Glycoluril
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
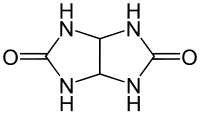
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Glycoluril | |||||||||||||||
| other names |
Tetrahydroimidazo [4,5- d ] imidazole-2,5 (1 H , 3 H ) -diones |
|||||||||||||||
| Molecular formula | C 4 H 6 N 4 O 2 | |||||||||||||||
| Brief description |
odorless white powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 142.116 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.8 g cm −3 |
|||||||||||||||
| Melting point |
~ 300 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Glycoluril is a bicyclic condensation product made from one molecule of glyoxal and two molecules of urea and is an odorless white powder. Strong intermolecular hydrogen bridges cause the high melting point of approx. 300 ° C, at which glycoluril decomposes. Glycoluril is the basic building block for the macrocyclic, cage-like cucurbiturils , which, like the similarly built cyclodextrins and calixarenes, can accommodate small molecules in their cavity.
Extraction and presentation
Glycoluril, by sodium amalgam - reduction of allantoin are obtained. Glycoluril is produced on an industrial scale by reacting 40% aqueous glyoxal with urea in approx. 90% yield.
Chemical properties
The four amide-like and therefore acidic hydrogen atoms of glycoluril are accessible to a number of chemical reactions, such as B. the substitution by halogens or the reaction with formaldehyde .
use
Glycoluril is used as a raw material for tetrachloro- and tetrabromoglycoluril , which are used as biocides in water treatment, for swimming pool disinfection and as an enhancer for slime control agents in paper and cardboard manufacture.
Glycoluril was discussed as a nitrogen fertilizer with delayed N release, but was not widely used because of its high cost.
Glycoluril is converted with excess formaldehyde to tetramethylolglycoluril , which releases formaldehyde with a delay and is therefore used as a biocide in water-based paints, in liquid detergents and in care and cleaning agents (in concentrations of 0.1%). It is also used as a crosslinker for polymers containing hydroxyl groups , as an industrial fungicide and as an accelerator in cements.
Glycoluril is converted into tetraacetylglycoluril (TAGU) by reaction with acetic anhydride , which has not found widespread use as a bleach activator for sodium percarbonate in solid detergent preparations due to its slow biodegradability .
The reaction with nitrating acid leads to the explosive tetranitroglycoluril (TNGU).
Individual evidence
- ↑ a b c d e f g Glycoluril data sheet at the National Institute of Environmental Health Sciences , accessed on July 29, 2017 (PDF; 34 kB).
- ↑ a b Glycoluril data sheet from Sigma-Aldrich , accessed on April 3, 2011 ( PDF ).
- ^ Frank B. Slezak, Henry Bluestone, Thomas A. Magee, John H. Wotiz: Preparation of Substituted Glycolurils and Their N-Chlorinated Derivatives . In: The Journal of Organic Chemistry . tape 27 , no. 6 , 1962, pp. 2181-2183 , doi : 10.1021 / jo01053a069 .
- ↑ T. Shimidzu: Glycoluril as a Slow Release Nitrogen Fertilizer . In: Soil Science and Plant Nutrition . tape 33 , no. 2 , 1987, ISSN 0038-0768 , pp. 291-298 , doi : 10.1080 / 00380768.1987.10557574 .
- ↑ Use of formaldehyde or formaldehyde-releasing agents in care and cleaning agents in private households (PDF; 53 kB), lecture at the BfR specialist event "Re-evaluation of formaldehyde - BfR's contribution to consumer protection"
- ↑ Uri Zoller: Handbook of detergents. Part E, Applications . CRC Press, Boca Raton, FL 2008, ISBN 978-1-4200-1816-5 , Chapter 16: Application of Surfactants in Environmental Remediation .
- ^ JK Agrawal, RD Hodgson: Organic chemistry of explosives . John Wiley & Sons, Chichester 2007, ISBN 978-0-470-02967-1 , pp. 278 .
