Hantzsch pyrrole synthesis
The Hantzsch pyrrole synthesis ( Hantzsch pyrrole synthesis ), also known as the Hantzsch pyrrole synthesis , is a name reaction in organic chemistry . It was named after the German chemist Arthur Hantzsch (1857-1935), who published it in 1890. It is used for the synthesis of pyrrole and its derivatives .
The reaction is related to the Paal-Knorr synthesis and the Knorr-pyrrole synthesis .
Overview
During the synthesis, β-keto esters ( black ) react with an amine ( blue ) and then with closure of a ring with an α-halo ketone ( green ) to form a pyrrole derivative:
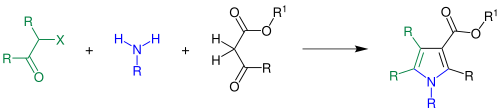 R = hydrogen , alkyl group , aryl group ; R 1 = alkyl group, aryl group; X = chlorine , bromine , iodine
R = hydrogen , alkyl group , aryl group ; R 1 = alkyl group, aryl group; X = chlorine , bromine , iodine
Instead of an amine, ammonia can also be used for the reaction. A pyrrole derivative which is unsubstituted on the nitrogen atom is then formed.
mechanism
The mechanism is illustrated with a general β-keto ester, an α-halo ketone and a primary amine.
At the beginning, the β-ketoester 1 reacts with a primary amine via several intermediate stages to form an enamine 2 . Then the enamine attacks the α-haloketone in the course of a nucleophilic substitution and a halide is split off. This halide splits off a hydrogen from nitrogen 3 and leaves the reaction as hydrogen halide (shown here as HX ). This creates an imine 4 whose imine group is rotated 5 in the next step . The imine is isomerized to the enamine tautomer 6 . Then the carbon atom of the carbonyl group 6 is attacked by the amino group and thus the five-membered ring 7 is closed. After a proton 7 has been rearranged , water is split off from molecule 8 . The product 9 is formed in the form of a pyrrole derivative.
Modifications
Instead of the three original starting materials, the reaction can also be carried out between an enamine of the β-keto ester and an α-halo ketone. In addition, through the choice of starting materials, the reaction can be used to synthesize indole or carbazole derivatives . For example, reacting o -amino acetophenone and α-chloroacetophenone to 2- benzoyl -3- methyl indole:
Individual evidence
- ↑ a b c d e f Z. Wang: Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 1326-1327.
- ↑ A. Hantzsch: New mode of formation of pyrrole derivatives . In: Reports of the German Chemical Society . tape 23 , no. 1 , 1890, p. 1474–1476 , doi : 10.1002 / cber.189002301243 .
- ↑ W. Uhl, A. Kyriatsoulis: Name and Keyword reactions in organic chemistry . 5. through Edition, Springer Fachmedien, Wiesbaden 1984, ISBN 978-3-663-02003-5 , pp. 230-232.
- ^ F. Feist: Studies in the furan and pyrrole group . In: Reports of the German Chemical Society . tape 35 , no. 2 , 1902, pp. 1537–1544 , doi : 10.1002 / cber.19020350263 .
- ^ JJ Li: Name Reactions. A Collection of Detailed Reaction Mechanisms . 3rd expanded edition, Springer-Verlag, Berlin / Heidelberg 2006, ISBN 978-3-540-30030-4 , pp. 283-284.
- ↑ CD Jones, T. Suarez: Direct synthesis of 2-acylindoles . In: J. Org. Chem. Band 37 , no. 23 , 1972, p. 3622-3623 , doi : 10.1021 / jo00796a012 .

