Hexanitroethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Hexanitroethane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 N 6 O 12 | |||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 300.0544 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.85 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
135.5 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Hexanitroethane is an aliphatic nitro compound . In the molecule, all six possible are substituent positions in each case by a nitro group replaced . As a highly nitrated compound, it acts as a strong oxidizing agent and is an explosive .
Presentation and extraction
The first production was described in 1914 by Wilhelm Will . This is achieved by reacting the potassium salt of tetranitroethane with nitric acid .
The potassium salt of tetranitroethane can be obtained in a synthesis sequence starting from tribromonitromethane. The first step is a dimerization to tetrabromodinitroethane.
In the second step there is a nucleophilic substitution with potassium nitrite to give dibromotetranitroethane.
The tetranitroethane salt then results from the further reaction with potassium cyanide .
A practical synthesis for industrial production starts from furfural , which is oxidatively brominated to mucobromic acid (2,3-dibromomaldehydic acid) in the first step with ring opening . Alternatively, furan-2-carboxylic acid can also be used as the starting material. In the next step , the mucobromic acid is reacted with potassium nitrite at temperatures just below room temperature to form the dipotassium salt of 2,3,3-trinitropropioaldehyde. The target compound is obtained by nitrating this salt with nitric acid and sulfuric acid at −60 ° C.
properties
Hexanitroethane can occur in two polymorphic crystal forms. Crystal form II is present below a temperature of 18 ° C. At the transition point at 18 ° C, the formation of crystal form I takes place with a conversion enthalpy of 12.4 kJ mol −1 . The crystal form I then melts at 135.5 ° C.
Hexanitroethane is an explosive substance within the meaning of the Explosives Act . Important explosion indicators are:
Table with important explosion-relevant properties: Oxygen balance 42.7% Nitrogen content 28.01% Normal gas volume 678 l kg −1 Explosion heat 3020 kJ kg −1 Specific energy 813 kJ kg −1 (68.1 mt / kg) Lead block bulge 24.5 cm 3 g −1 Detonation velocity 4950 m s −1 Deflagration point 175 ° C Sensitivity to impact 10 Nm Rubbing sensitivity Decomposition with 253 N pin load
The thermal decomposition was detected on the solid and in solution from 60 ° C. Above 140 ° C the decomposition can take place explosively. The decomposition proceeds as a first-order reaction . The reaction rate is significantly higher in solution than in the solid. The following equation can be formulated for the decomposition of the solid.
During the decomposition in solution, tetranitroethylene is formed in the first step , which via its Diels-Alder adducts z. B. can be detected with anthracene or cyclopentadiene .
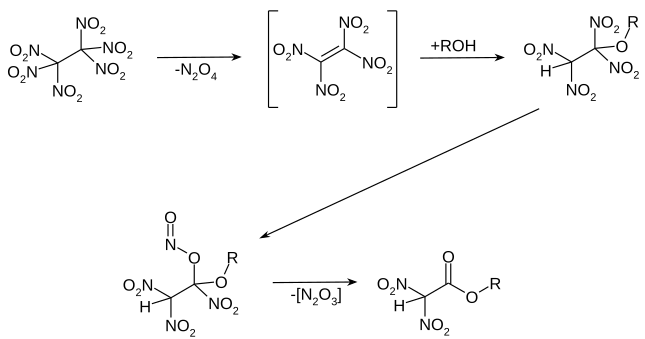
When reacted in alcohols under reflux, there is an addition to the tetranitroethylene formed as an intermediate. The dinitroalkoxyalkyl structure formed is not stable and, after rearrangement of the nitro group to form the nitrous acid ester and the elimination of nitrogen oxides, forms the corresponding ester of dinitroacetic acid.
The dinitroacetic acid ester slowly dimerizes on storage to give the corresponding 3,4-bis (alkoxycarbonyl) furazan-2-oxide. At higher temperatures, the dinitroacetic ester can be saponified and decarboxylated to dinitromethane .
use
Hexanitroethane can be used in propellants as an oxygen-compensating additive.
Individual evidence
- ↑ a b c Urbanski, T .: Chemistry and Technology of Explosives, Vol. 1 , Pergamon Press / PWN - Polish Scientific Publishers 1964, p. 596.
- ↑ a b c d e f g h i j k l m n Köhler, J .; Meyer, R .; Homburg, A .: Explosivstoffe , tenth, completely revised edition. Wiley-VCH, Weinheim 2008, p. 165, ISBN 978-3-527-32009-7 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Will, W .: About the hexanitro-ethane in Chemical Reports 47 (1914) 961-965, doi : 10.1002 / cber.191404701154 .
- ↑ a b c Gallaghan, JA; Lomond, B .; Reed, WL: Synthesis of Hexanitroethane , Patent US 3,101,379 (1963). Google Patents
- ↑ Taylor, GA: Mucobromic Acid. In: Organic Syntheses , Coll. Vol. 4 (1963), p. 688, doi : 10.1002 / 0471264180.os900.13 ( PDF ).
- ↑ Allen, CFH; Spangler, FW: Mucobromic Acid In: Organic Syntheses . 27, 1947, p. 60, doi : 10.15227 / orgsyn.027.0060 ; Coll. Vol. 3, 1955, p. 621 ( PDF ).
- ↑ a b Krien, G .; Light, HH; Trimborn, F .: A phase transition of hexanitroethane (HNE) in Explosivstoffe 9 (1970) 203-207.
- ↑ a b c Marshall, HP; Borgardt, FG; Noble, Jr. P .: Thermal Decomposition of Hexanitroethane in J. Phys. Chem. 69 (1965) 25-29, doi : 10.1021 / j100885a007 .
- ↑ PG Urben; MJ Pitt: Bretherick's Handbook of Reactive Chemical Hazards . 8th Edition, Vol. 1, Butterworth / Heinemann 2017, ISBN 978-0-08-100971-0 , pp. 240–241.
- ↑ Griffin, TS; Baum, K .: Tetranitroethylene. In Situ Formation and Diels-Alder Reactions in J. Org. Chem. 45 (1980) 2880-2883, doi : 10.1021 / jo01302a024 .
- ↑ a b c Tseng, D .; Baum, K .: Reactions of Hexanitroethane with alcohols in J. Org. Chem. 48 (1983) 5384-5385, doi : 10.1021 / jo00174a053 .
- ↑ a b Grakauskas, V .; Guest, AM: Dinitromethane in J. Org. Chem. 43 (1978) 3485-3488, doi : 10.1021 / jo00412a014 .