Dinitromethane
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Dinitromethane | ||||||||||||
| Molecular formula | CH 2 N 2 O 4 | ||||||||||||
| Brief description |
colorless liquid with a faint, pleasant odor |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 106.0376 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| density |
1.524 g cm −3 (20 ° C) |
||||||||||||
| boiling point |
39-40 ° C (2 torr ) |
||||||||||||
| pK s value |
3.57 (20 ° C) |
||||||||||||
| Refractive index |
1.4480 (20 ° C) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Thermodynamic properties | |||||||||||||
| ΔH f 0 |
−104.9 kJ / mol |
||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||
Dinitromethane is the disubstituted representative of the series of nitromethanes with nitromethane , dinitromethane, trinitromethane and tetranitromethane as well as the simplest geminal dinitroalkyl compound. The connection is increasingly unstable above room temperature. Safe handling is more likely in the form of their alkali salts .
history
A first, uncertain source of the production of dinitromethane dates back to 1878, when dinitromethane is said to have been formed in a violent reaction from acetone and concentrated nitric acid . The first preparations of the potassium salt were made by Villiers in 1884 and Duden in 1893 in low yields from bromodinitromethane, which was obtained by heating 2,4,6-tribromaniline with concentrated nitric acid. A 1951 by Feuer et al. published two-stage synthesis variant is based on nitromethane, which is first chlorinated to form chloronitromethane. The potassium salt is created by nucleophilic substitution using potassium nitrite in the basic medium. However, the yield of this variant is only 23%.
The action of concentrated or moderately diluted acids on the potassium salt led to decomposition at room temperature with vigorous formation of nitrous gases. The dinitromethane can only be released in the cold in an ethereal solution by carefully adding dilute sulfuric acid in portions.
Presentation and extraction
One synthesis is based on monomethyl malonate, which is converted into methyl 2,2-dinitroacetate by oxidative nitration with decarboxylation. The second decarboxylation step takes place in the basic hydrolysis of this ester using sodium hydroxide solution, the sodium salt of dinitromethane being formed first.
A more recent synthesis starts with the nitration of barbituric acid under mild conditions. The resulting 5,5-dinitrobarbituric acid can already be hydrolyzed nucleophilically with water, with the formation of 2,2-dinitroacetylurea with the elimination of carbon dioxide . This can be decomposed in the heat with potassium hydroxide to the potassium salt of dinitromethane and urea . The overall yield of this synthesis variant is 80%.
Dinitromethane is also a by-product of the synthesis of diaminodinitroethylene . The synthesis starts from 2,6-dihydroxy-4-methylpyrimidine , which is converted to a tetranitro intermediate by nitration in nitrating acid . This is then hydrolytically split into dinitromethane, diaminodinitroethylene and carbon dioxide .
Diaminodinitroethylene has acidic properties. Deprotonation occurs in the presence of bases. The potassium salt can be isolated as a white, crystalline solid by reaction with potassium hydroxide solution at low temperatures. Heating to 70 ° C with potassium hydroxide leads to a basic hydrolysis, with the potassium salt of dinitromethane and urea being formed.
Dinitromethane can be released from the potassium salt by carefully introducing hydrogen fluoride into an ethereal solution of the salt at 0-5 ° C.
properties
Dinitromethane is a colorless to yellowish oil, depending on its purity. The compound is unstable and slowly decomposes at room temperature. At 0 ° C the connection is stable for months. Distillation was carried out in vacuo at 2 torr with a boiling point of 39-40 ° C and at 4 torr at 52-53.5 ° C. However, it is not recommended because of its ability to decompose explosively. A heat of detonation of 6814 kJ mol −1 was estimated for the compound . By the -M effect of the nitro groups is dinitromethane CH-acid with a pK s value of 3.57 a strong acid medium. Its acidity is comparable to that of formic acid (pK s 3.75). Two tautomeric structures can be formulated for dinitromethane . The equilibrium is on the side of the CH-acidic structure. Quantum chemical calculations show a difference in free energy of 19.6 kJ mol −1 to the N-OH acidic structure. The 1 H-NMR spectrum shows only a single signal at 3.9 ppm for the CH function. As expected, this value lies between those for nitromethane with 5.72 ppm and trinitromethane with 2.48 ppm. The comparison of quantum-chemically calculated and experimental IR and Raman spectra confirms the CH-acidic structure.
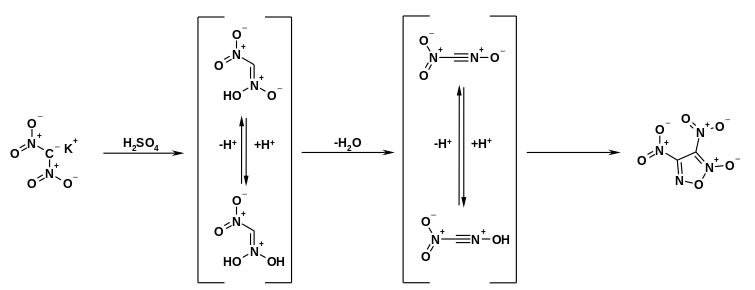
In a strongly acidic medium, the equilibrium can be stabilized by a protolysis equilibrium and shifted in the direction of the N-OH-acidic structure. The cyclodimerization product dinitrofuroxane can be obtained by introducing the potassium salt of dinitromethane into concentrated or fuming sulfuric acid . The formation of this dimer takes place via the intermediates of the protonated N-OH-acidic structure of dinitromethane and the nitroformonitrile oxide resulting therefrom through elimination of water.
The salts of dinitromethane are characterized by a significantly higher stability compared to the free compound. In the case of the potassium salt, for example, explosive decomposition is only observed from 200 ° C. Potassium carbonate , water , carbon dioxide , nitrogen monoxide and nitrogen were detected as decomposition products . The salts of organic bases begin to decompose around 100 ° C. For the piperazinium salt, a decomposition from 90 ° C., for the formamidinium salt from 125 ° C. and the guanidinium salt from 175 ° C. was observed.
Chemical reactions are usually based on the potassium or sodium salt of dinitromethane. The anion acts as a good nucleophile. It reacts with formaldehyde to form 2,2-dinitropropane-1,3-diol.
The reaction with dialkyl or diaryl sulfoxides gives the corresponding sulfanylidenedinitromethanes.
use
Despite the explosiveness, the compound is not used as an explosive because of its poor handleability. The salts of dinitromethane can be used as building blocks for heterocycle syntheses. The salts with alkylated imidazoles can be used as ionic liquid .
Individual evidence
- ↑ a b c d e f g h Legin, G. Ya .; Okhlobystina, LV; Fainzilberg, AA: Preparation of pure dinitromethane and its properties . In: Russian Chemical Bulletin . 14, No. 12, 1965, pp. 2190-2191. doi : 10.1007 / BF00846018 .
- ↑ a b Adolph, HG; Kamlet, MJ: Fluoronitroaliphatics. I. The Effect of α Fluorine on the Acidities of Substituted Nitromethanes . In: Journal of the American Chemical Society . 88, No. 20, 1966, pp. 4761-4763. doi : 10.1021 / ja00972a065 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-19.
- ↑ Chancel: Compt. Rend. 86 (1878) 1405 and annual report for 1878, 694
- ↑ a b c d e f Duden, P .: About dinitromethane . In: Reports of the German Chemical Society . 26, No. 3, 1893, pp. 3003-3011. doi : 10.1002 / cber.189302603135 .
- ^ A. Villiers: Bull. Soc. Chim. Fr. 41 (1884) 281 and 43 (1886) 322.
- ↑ a b c Feuer, H .; Bachmann, GB; Kispersky, JP: A New Preparation of Potassium Dinitromethane and its Conversion to 2,2-Dinitro-1,3-propanediol . In: Journal of the American Chemical Society . 73, No. 3, 1951, p. 1360. doi : 10.1021 / ja01147a511 .
- ^ Grakauskas, V .; Guest, AM: Dinitromethane . In: Journal of Organic Chemistry . 43, No. 18, 1978, pp. 3485-3488. doi : 10.1021 / jo00412a014 .
- ↑ Langlet, A .; Latypov, NV; Wellmar, U .; Goede, P .; Bergman, J .: Synthesis and reactions of 5,5-dinitrobarbituric acid . In: Tetrahedron Letters . 41, No. 12, 2000, pp. 2011-2013. doi : 10.1016 / S0040-4039 (00) 00086-1 .
- ↑ Latypov, NV; Johansson, M .; Holmgren, E .; Sizova, EV; Sizov, VV; Bellamy, AJ: On the Synthesis of 1,1-Diamino-2,2-dinitroethene (FOX-7) by Nitration of 4,6-Dihydroxy-2-methylpyrimidine . In: Organic Process Research and Development . 11, No. 1, 2007, pp. 56-59. doi : 10.1021 / op068010t .
- ↑ Bellamy, AJ: FOX-7 (1,1-diamino-2,2-dinitroethene) . In: Structure and Bonding . 125, 2007, pp. 1-33. doi : 10.1007 / 430_2006_054 .
- ^ Zeman, S .: New application of kinetic data of the low-temperature thermolysis of nitroparaffins . In: Thermochimica Acta . 261, 1995, pp. 195-207. doi : 10.1016 / 0040-6031 (95) 02325-V .
- ^ Zeman, S. Modified Evans-Polanyi-Semenow relationship in the study of chemical micromechanism governing detonation initiation of individual energetic materials . In: Thermochimica Acta . 384, No. 1-2, 2002, pp. 137-154. doi : 10.1016 / S0040-6031 (01) 00787-0 .
- ^ Brand, H .; Liebman, JF; Schulz, A .: Cyano-, Nitro- and Nitrosomethane Derivatives: Structures and Gas-Phase Acidities . In: European Journal of Organic Chemistry . 2008, No. 27, 2008, pp. 4665-4675. doi : 10.1002 / ejoc.200800583 .
- ↑ a b Hofmann, W .; Stefaniak, L .; Urbanski, T .; Witanowski, M .: Proton Magnetic Resonance Study of Nitroalkanes . In: Journal of the American Chemical Society . 86, No. 4, 1964, pp. 554-558. doi : 10.1021 / ja01058a005 .
- ↑ Tafipolsky, MA; Tokmakov, IV; Shlyapochnikov, VA: Structure and vibrational spectra of dinitromethane and trinitromethane . In: Journal of Molecular Structure . 510, No. 1-3, 1999, pp. 149-156. doi : 10.1016 / S0022-2860 (99) 00080-0 .
- ↑ Edwards, JT; Tremaine, PH: The Meyer Reaction of Phenylnitromethane in Acid. III. The tautomerization to the aci form . In: Canadian Journal of Chemistry . 49 (21), 1971, pp. 3493-3501, doi : 10.1139 / v71-584 .
- ↑ a b Ovchinnikov, IV; Makhova, NN; Khmelnitskii, LI: Nitroformonitril oxide 2nd generation of nitroformonitrile oxide as an intermediate for the preparation of dinitrofuroxan . In: Russian Chemical Bulletin . 44, No. 4, 1995, pp. 702-706. doi : 10.1007 / BF00698507 .
- ↑ Jalový, Z .; Ottis, J .; Růžička, A .; Lyčka, A .; Latypov, NV: Organic salts of dinitromethane . In: Tetrahedron . 65, No. 34, 2009, pp. 7163-7170. doi : 10.1016 / j.tet.2009.06.014 .
- ↑ Shevelev, SA et al. in Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science (English Translation), 1976, Vol. 25, p. 1906-1909.
- ↑ Shitov, OP et al. in Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science (English Translation), 1977, Vol. 26, p. 214-217.
- ^ Brand, H .; Liebman, JL; Schulz, A .; Mayer, P .; Villinger, A .: Nonlinear, Resonance-Stabilized Pseudohalogenides: From Alkali Methanides to Ionic Liquids of Methanides . In: European Journal of Inorganic Chemistry . 2006, No. 21, 2006, pp. 4294-4308. doi : 10.1002 / ejic.200600668 .