Iclaprim
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Iclaprim | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
Inhibition of bacterial dihydrofolate reductase |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Iclaprim (intended trade name Iclaprim Arpida ® ; manufacturer Arpida ) is an antibiotic and belongs to the group of diaminopyrimidines . Iclaprim is in the approval process and is to be approved for the treatment of complex skin and skin structure infections (cSSSI).
Clinical information
Areas of application and spectrum of activity
Iclaprim is an antibiotic and belongs to the group of diaminopyrimidines . It acts as a potent inhibitor of bacterial dihydrofolate reductase , like trimethoprim , another representative of this class of substances. Iclaprim, on the other hand, is still effective against many trimethoprim-resistant pathogen strains. In in vitro studies , Iclaprim shows a high level of effectiveness against the most clinically relevant gram-positive bacteria . The spectrum of activity includes resistant strains isolated from patients with severe MRSA and VRSA infections. Opposite anaerobes Iclaprim shows little effect.
- Spectrum of sensitive germs
- Staphylococci , including Staphylococcus aureus including multi-resistant strains (MRSA),
- Enterococci including vancomycin-resistant strains (VRE),
- Streptococci including penicillin-resistant strains.
- Clinical application
- Iclaprim is to be approved for the treatment of complex skin and skin structure infections (cSSSI).
Pharmacological properties
Mechanism of action (pharmacodynamics)
Iclaprim works by inhibiting bacterial dihydrofolate reductase without significantly inhibiting the human enzyme. As a result, there is an inhibition of the synthesis of DNA building blocks and cell death.
Absorption and distribution in the body (pharmacokinetics)
In clinical trials, Iclaprim is given either intravenously or orally . When administered orally, the bioavailability is about 40%. With the recommended dose of 160 mg and oral administration, the maximum plasma level of 0.5 µg / ml is reached 90 minutes after ingestion. With intravenous administration of 0.4 and 0.8 mg / kg body weight, plasma concentrations of 0.37 and 0.87 µg / ml are achieved. The plasma half-life is about two hours.
Other Information
Iclaprim was in the fast-track approval process of the American Food and Drug Administration (FDA); approval was not granted for the time being in January 2009.
Stereochemistry
Iclaprim contains a stereocenter and consists of two enantiomers. This is a racemate , i.e. a 1: 1 mixture of ( R ) - and ( S ) -form:
| Enantiomers of phantolide | |
|---|---|
 CAS number: 1208116-65-7 |
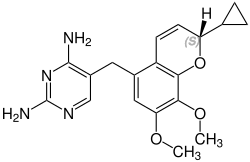 CAS number: 1208116-66-8 |
See also
literature
- Ernst Mutschler among others: Mutschler - drug effects textbook of pharmacology and toxicology . 9th edition. Scientific Verlagsgesellschaft, Stuttgart 2008, ISBN 978-3-8047-1952-1 .
- P. Schneider, S. Hawser, K. Islam: Iclaprim, a novel diaminopyrimidine with potent activity on trimethoprim sensitive and resistant bacteria . In: Bioorg Med Chem Lett . tape 13 , no. December 23 , 2003, pp. 4217-4221 , PMID 14623005 ( tufts.edu [PDF]).
- A. Morgan, C. Cofer, DL Stevens: Iclaprim: a novel dihydrofolate reductase inhibitor for skin and soft tissue infections. In: Future Microbiol. 4, March 2009, pp. 131-144, PMID 19257839 , doi: 10.2217 / 17460913.4.2.131 .
Individual evidence
- ↑ a b Registration dossier on 2,4-Pyrimidinediamine, 5 - [(2-cyclopropyl-7,8-dimethoxy-2H-1-benzopyran-5-yl) methyl] - ( GHS section ) at the European Chemicals Agency (ECHA), accessed on June 10, 2020.
- ↑ WJ Peppard, CD Schuenke: Iclaprim, a diaminopyrimidine dihydrofolate reductase inhibitor for the potential treatment of antibiotic-resistant staphylococcal infections . In: Curr Opin Investig Drugs . tape 9 , no. 2 , February 2008, p. 210-25 , PMID 18246524 .
Web links
- Staphylococci and MRSA - information from the Robert Koch Institute