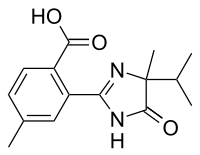
Imazamethabenz
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Mixture of isomers | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Imazamethabenz | |||||||||||||||
| other names |
2- (4,5-dihydro-4-methyl-4- (1-methylethyl) -5-oxo-1 H -imidazol-2-yl) -4 / 5-methylbenzoic acid |
|||||||||||||||
| Molecular formula | C 15 H 18 N 2 O 3 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 274.32 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
144-153 ° C |
|||||||||||||||
| solubility |
hardly soluble in water (1.1 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Imazamethabenz is a chemical compound from the imidazolinone group .
Imazamethabenz and its methyl ester imazamethabenz-methyl are used as herbicides .
Synthesis and properties
Imazamethabenz can be obtained by chlorination of 2,5-dimethylbenzoic acid ethyl ester and subsequent reaction with 2-amino-2,3-dimethylbutanamide and with sodium hydroxide solution .
Imazamethabenz is chiral . The technical product is typically a mixture of rac -5-methyl-2 - [(4 R ) -4-methyl-5-oxo-4- (propan-2-yl) -4,5-dihydro-1 H -imidazole -2-yl] benzoic acid and rac -4-methyl-2 - [(4 R ) -4-methyl-5-oxo-4- (propan-2-yl) -4,5-dihydro-1 H -imidazole- 2-yl] benzoic acid in a ratio of 3: 2.
use
Imazamethabenz as well as its methyl ester imazamethabenz-methyl are used as herbicides and used for selective post-emergence control of weeds in cereals and sunflowers.
In 2005, the EU Commission decided not to include imazamethabenz in the list in Annex I of Directive 91/414 / EEC of permissible active ingredients in plant protection products .
In Germany, Austria and Switzerland, no pesticides with this active ingredient are permitted.
Individual evidence
- ↑ a b c d e Entry on Imazamethabenz in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on November 15, 2013.
- ^ Robert Irving Krieger, Handbook of Pesticide Toxicology: Principles . Academic Press, 2001, ISBN 978-0-12-426260-7 , pp. 1642 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 442 ( limited preview in Google Book search).
- ↑ External identifiers of or database links for Benzoic acid, 2- [4,5-dihydro-4-methyl-4- (1-methylethyl) -5-oxo-1H-imidazol-2-yl] -4 (or 5) -methyl-, methyl ester : CAS number: 81405-85-8, EC number: 617-231-3, ECHA info card: 100.129.120 , PubChem : 54744 , Wikidata : Q61971747 .
- ↑ Data sheet imazamethabenz-methyl, PESTANAL at Sigma-Aldrich , accessed on October 16, 2016 ( PDF ).
- ↑ 2005/303 / EC: Commission decision of March 31, 2005 on the non-inclusion of cresylic acid, dichlorophene, imazamethabenz, kasugamycin and polyoxin in Annex I of Council Directive 91/414 / EEC and the revocation of the authorizations for plant protection products with these active substances
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Imazamethabenz in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 18, 2016.