Imazaquin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Racemate | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Imazaquin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 17 H 17 N 3 O 3 | |||||||||||||||
| Brief description |
|
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 311.34 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.41 g cm −3 (bulk density) |
|||||||||||||||
| Melting point |
219-224 ° C |
|||||||||||||||
| boiling point |
Decomposition from 250 ° C |
|||||||||||||||
| solubility |
very sparingly soluble in water (60–120 mg · l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Imazaquin is a 1: 1 mixture of two stereoisomeric chemical compounds from the group of imidazolinones .
Extraction and presentation
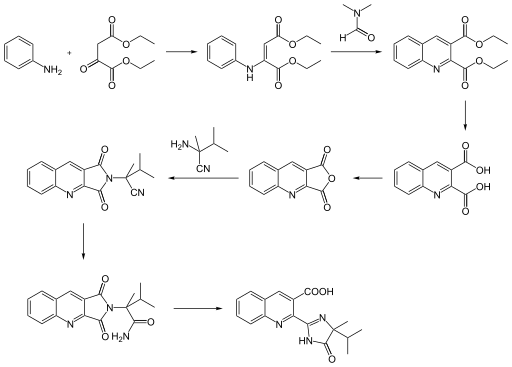
Imazaquin can be obtained from o- aminobenzaldehyde or aniline through a multi-stage reaction .
use
Imazaquin is used as a herbicide . It is used for pre- and post-emergence control of a wide range of weeds including annual and perennial grasses and dicotyledonous plants. Imazaquin was introduced in 1986 by American Cyanamid in the USA, where it is mainly used in soy cultivation. The effect is based on the inhibition of acetolactate synthase . It is the most lipophilic of the imidazolinones and is therefore absorbed into the plant the fastest.
Admission
In some EU countries, plant protection products with this active ingredient are approved, but not in Germany, Austria or Switzerland.
Individual evidence
- ↑ a b c d e Entry on Imazaquin in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on February 28, 2014.
- ↑ a b Entry on Imazaquin. In: Römpp Online . Georg Thieme Verlag, accessed on May 15, 2014.
- ↑ a b Imazaquin data sheet at Sigma-Aldrich , accessed on May 21, 2017 ( PDF ).
- ↑ Thomas A. Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5 , pp. 445 ( limited preview in Google Book search).
- ^ Terence Robert Roberts, DH Hutson: Metabolic pathways of agrochemicals, Volume 2 . Royal Soc of Chemistry, 1999, ISBN 978-0-85404-499-3 , pp. 368 ( limited preview in Google Book search).
- ↑ Wolfgang Krämer, Ulrich Schirmer, Peter Jeschke, Matthias Witschel: Modern Crop Protection Compounds: Herbicides, Volume 1 . Wiley-VCH, 2011, ISBN 978-3-527-32965-6 , pp. 92 ( limited preview in Google Book search).
- ↑ Directorate-General for Health and Food Safety of the European Commission: Entry on imazaquin in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 25, 2016.
- ↑ Implementing Regulation (EU) No. 1100/2011 of the Commission of October 31, 2011 amending Implementing Regulation (EU) No. 540/2011 with regard to the conditions for the approval of the active substances dicamba, difenoconazole and imazaquin (PDF) .