Indoxacarb
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
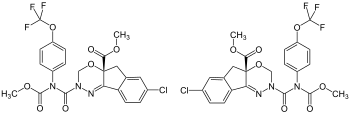
|
|||||||||||||||||||
| 1: 1 mixture of ( R ) -form (left) and ( S ) -form (right) | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Indoxacarb | ||||||||||||||||||
| other names |
( RS ) -Methyl-7-chloro-2,3,4a, 5-tetrahydro-2- [methoxycarbonyl- (4-trifluoromethoxyphenyl) carbamoyl] indeno [1,2- e ] [1,3,4] oxadiazine-4a carboxylate |
||||||||||||||||||
| Molecular formula | C 22 H 17 ClF 3 N 3 O 7 | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 527.83 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.44 g cm −3 |
||||||||||||||||||
| Melting point |
88.1 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Indoxacarb is an active ingredient for crop protection and a 1: 1 mixture (racemate) of two chemical compounds from the group of oxadiazines .
presentation
Indoxacarb can be obtained by enantioselective hydroxylation of the intermediate 2-carboalkoxy-1-indanone, catalyzed by a cinchona alkaloid.
properties
Indoxacarb is a colorless solid that is insoluble in water. The technical product is a 3: 1 mixture of the ( S ) -enantiomer, which is effective as an insecticide, and the ineffective ( R ) -enantiomer.
use
Indoxacarb is used as an insecticide and was approved in the USA for the manufacturer DuPont in 2000 . It is used to control Lepidoptera pests in the larval stage in apples, pears, cabbage vegetables, corn, lettuce and vegetables.
Admission
The active ingredient indoxacarb was added to the list of plant protection active ingredients approved in the European Union with effect from April 1, 2006 .
Pesticides with this active ingredient are approved in Switzerland, Austria and Germany ( Steward in vegetable, fruit and viticulture, Avaunt in rapeseed cultivation).
Indoxacarb has been approved as a biocidal active ingredient in the European Union since the beginning of 2010. In Germany an application was made for approval as a biocide against cockroaches and ants.
Mode of action
Indoxacarb is a proinsecticide, the actual active ingredient is only created by N -decarboxymethylation by esterases . This works by blocking the voltage-activated sodium channels in the insect's nervous system. Since indoxacarb is only activated by metabolism, it is particularly effective against insect strains that are already pyrethroid- resistant.
Individual evidence
- ↑ a b c d e f EPA: Pesticide Fact Sheet
- ↑ a b c d Data sheet Indoxacarb, PESTANAL at Sigma-Aldrich , accessed on December 30, 2019 ( PDF ).
- ↑ Entry on indoxacarb in the GESTIS substance database of the IFA , accessed on December 30, 2019(JavaScript required) .
- ↑ Entry on indoxacarb (ISO) in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 30, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Toward the Manufacture of Indoxacarb, Synthesis and Chemistry of Agrochemicals VI, chap. 17, 2002, pp. 178-185, doi : 10.1021 / bk-2002-0800.ch017 .
- ↑ Directive 2006/10 / EC of the Commission of January 27, 2006 amending Council Directive 91/414 / EEC to include the active substances forchlorfenuron and indoxacarb
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on indoxacarb in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 12, 2016.
- ↑ Directive 2009/87 / EC of 29 July 2009 amending Directive 98/8 / EC of the European Parliament and the Council to include the active substance indoxacarb in Annex I .
- ↑ Alexander Grube: Total synthesis of new spinosyn analogues . Cuvillier, Göttingen 2007, ISBN 3-86727-439-8 , p. 28 ( limited preview in Google Book search).
- ↑ Robert Krieger: Hayes' Handbook of Pesticide Toxicology . tape 1 . Elsevier, 2010, ISBN 3-89129-800-5 , pp. 106 (English, limited preview in Google Book Search).
- ↑ Bhupinder PS Khambay: pyrethroid Insecticides . (PDF; 144 kB) Pesticide Outlook - April 2002, p. 52.


