Potassium hexacyanidoferrate (III)
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
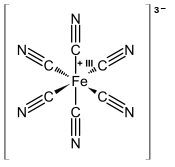
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium hexacyanidoferrate (III) | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | K 3 [Fe (CN) 6 ] | |||||||||||||||
| Brief description |
ruby red, monoclinic crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 329.26 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.85 g cm −3 |
|||||||||||||||
| Melting point |
Decomposition from 300 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Potassium hexacyanidoferrate (III) is a salt with the formula unit K 3 [Fe (CN) 6 ]. It is also known as potassium , ferricyanide , red potassium ferrocyanide , or Rotkali designated; According to the older IUPAC nomenclature, it is also known as potassium hexacyanoferrate (III). The aqueous solution has a yellow color and decomposes in the light to form iron (III) hydroxide (Fe (OH) 3 ).
origin of the name
The name blood liquor salt comes from the way it is made by alchemists . They heated blood with bone, horn and other proteinaceous substances in the presence of potash . The residue was leached with water. A salt then crystallized out of it, which was red (red blood liquor salt) or yellow ( yellow blood liquor salt ) depending on how much air was allowed in during heating .
Manufacturing
It is produced by oxidation of potassium hexacyanidoferrate (II) (K 4 [Fe (CN) 6 ]) with hydrogen peroxide (H 2 O 2 ) or electrochemically.
use
The salt is used as a mild oxidizing agent in organic syntheses . It is used in analytics to detect iron (II) ions. This creates the so-called Berlin blue .
Cyanotypes are made with potassium hexacyanidoferrate (III). It is also used in dyeing , as a steel hardener , as wood stain and in analog photography , e.g. B. as part of the Farmer's attenuator .
If handled improperly, potassium hexacyanidoferrate (III) can form the very toxic hydrocyanic acid (hydrogen cyanide) through various reactions . This danger is particularly present in reactions with strong acids under the influence of heat. Even in the case of contact with strong acids in the cold, hydrogen cyanide can be released under unfavorable circumstances, since potassium hexacyanidoferrate (III), in contrast to potassium hexacyanidoferrate (II), shows a weak ligand exchange between water and cyanide .
See also
- Potassium hexacyanidoferrate (II) (yellow blood liquor salt)
- Cyanide
- Cyanotype
- blueprint
Individual evidence
- ↑ a b Entry on Hexacyanidoferrate. In: Römpp Online . Georg Thieme Verlag, accessed on June 6, 2014.
- ↑ a b c d e f Entry on potassium hexacyanidoferrate (III) in the GESTIS substance database of the IFA , accessed on February 28, 2017(JavaScript required) .

![\ mathrm {Fe ^ {2 +} + [Fe (CN) _ {6}] ^ {3 -} \ \ rightleftharpoons \ Fe ^ {3 +} + [Fe (CN) _ {6}] ^ {4- }}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ba48a61dbef287bc9886697f83bbae98997a0c18)

