Letrozole
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
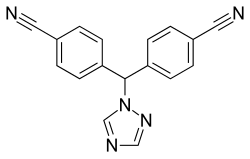
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Letrozole | ||||||||||||||||||
| other names |
4,4 '- (1 H -1,2,4-triazol-1-ylmethylene) bisbenzonitril |
||||||||||||||||||
| Molecular formula | C 17 H 11 N 5 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 285.30 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
181-183 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Letrozole is a drug from the group of aromatase inhibitors . It is taken orally. Letrozole was patented by Ciba-Geigy in 1989 under the brand name Femara and is used in the treatment of primary and metastatic breast cancer in postmenopausal women.
Mechanism of action
Letrozole inhibits excessive aromatization from androgens to estrogens. The effect and strength is similar to that of anastrozole .
Abuse as a doping agent
The active ingredient was placed on the prohibited list by the World Anti-Doping Agency (WADA) in 2008 . Possession of more than 75 mg is assessed as a "not small amount" according to the Medicines Act , according to the doping agent quantity regulation .
Side effects
- Hot flashes and rednesses (flushes)
- fatigue
- a headache
- nausea
- depressions
- sleep disorders
- Muscle and joint pain
literature
- The Black Book - Anabolic Steroids , 2010, p. 307, ISBN 978-3-00-020944-4
Individual evidence
- ↑ a b Data sheet Letrozole, ≥98% (HPLC) from Sigma-Aldrich , accessed on November 29, 2012 ( PDF ).
- ↑ a b Entry on Letrozole. In: Römpp Online . Georg Thieme Verlag, accessed on July 13, 2019.