Anastrozole
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
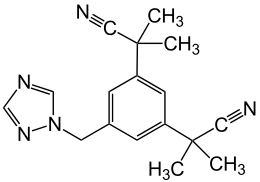
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Anastrozole | |||||||||||||||||||||
| other names |
2,2 '- [5- (1 H -1,2,4-triazol-1-ylmethyl) -1,3-phenylene] -bis (2-methylpropanenitrile) |
|||||||||||||||||||||
| Molecular formula | C 17 H 19 N 5 | |||||||||||||||||||||
| Brief description |
Solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 293.37 g · mol -1 | |||||||||||||||||||||
| solubility |
soluble in DMSO |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Anastrozole is a drug from the group of aromatase inhibitors . It is approved in Germany under the brand name Arimidex for the supportive treatment of hormone-dependent breast cancer in women after the menopause .
effect
The effects of anastrozole are based on the inhibition of the enzyme aromatase , which determines the conversion of androgens into the estrogens estrone and estradiol . By inhibiting this enzyme, the estrogen level in the blood plasma is lowered, which means that the tumor cells have fewer hormones available for growth. Anastrozole has no steroid structure and, in contrast to exemestane , reversibly inhibits aromatase. Furthermore, anastrozole has a direct effect on the tumor cells by also inhibiting the aromatase within the tumor cells.
In the prevention of breast cancer, anastrazole has been used successfully in postmenopausal women at high risk of breast cancer. In this group, anastrozole can cut the risk of developing breast cancer in half. Since breast cancer is now quite treatable, it remains questionable whether the possible side effects should really be accepted. Because prevention does not bring any survival advantage.
A single dose of 1 mg lowers the estrogen level by 70% in 7 days and by up to 80% in 14 days.
Anastrozole has no gestagenic, androgenic or estrogenic effects.
Use as a doping agent
Anastrozole is primarily used to prevent excessive aromatase activity and thus increased water and fat storage through testosterone administration . The active ingredient was placed on the prohibited list by the World Anti-Doping Agency (WADA).
Side effects
- Hot flashes and rednesses (flushes)
- fatigue
- a headache
- nausea
- depressions
- sleep disorders
- Worsening of cholesterol levels
- osteoporosis
synthesis
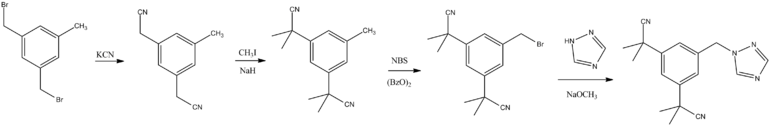
Anastrozole can be produced from α, α'-dibromomesitylene in a four-step process. In the first step, the α, α'-dibromommesitylene is converted to the corresponding dinitrile by nucleophilic substitution with potassium cyanide with the aid of a phase transfer catalyst , which is then exhaustively methylated with methyl iodide . With N- bromo succinimide , the corresponding benzyl bromide derivative is produced in a radical bromination, which is converted in the last step in the basic with 1,2,4-triazole to anastrozole.
Finished medicinal products
Arimidex ( D , A , CH and others), Asiolex, Lezole, Trozolet and various generics
Individual evidence
- ↑ a b c d Data sheet Anastrozole, ≥ 98% (HPLC) from Sigma-Aldrich , accessed on December 27, 2011 ( PDF ).
- ↑ N. Maass, W. Jonat: Aromatase inhibitors in the adjuvant therapy of breast cancer. In: The gynecologist. Volume 36, Number 2, 2003, pp. 103-109.
- ↑ K. Höffken: Aromatase Inhibitors of the 3rd Generation in the Therapy of Breast Cancer. In: The oncologist. Volume 5, Number 1, 1999, pp. 58-67.
- ↑ Jack Cuzick, Ivana Sestak, John F Forbes, Mitch Dowsett, Jill Knox, Simon Cawthorn, Christobel Saunders, Nicola Roche, Robert E Mansel, Gunter von Minckwitz, Bernardo Bonanni, Tiina Palva, Anthony Howell: Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomized placebo-controlled trial. In: The Lancet. 2013, S., doi: 10.1016 / S0140-6736 (13) 62292-8 .
- ↑ The Black Book - Anabolic Steroids. 2007, ISBN 978-3-00-020944-4 , p. 25.
- ↑ Substance Classification Booklet ( Memento of the original from September 27, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 703 kB), Canadian Center for Ethics in Sport, Version 4.0, January 2009.
- ↑ U.S. Patent 4,935,437.