Aromatase
| Aromatase | ||
|---|---|---|

|
||
| Belt model according to PDB 1TQA ( theoretical ) | ||
| Properties of human protein | ||
| Mass / length primary structure | 502 amino acids | |
| Secondary to quaternary structure | Membrane protein (ER) | |
| Identifier | ||
| Gene name | CYP19A1 | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.14.14.1 , oxidoreductase | |
| Response type | Hydroxylation, dehydration, deformylation | |
| Substrate | Androstenedione / Testosterone + 3NADPH / H + + 3O 2 | |
| Products | Estrone / estradiol + 3NADP + + 4H 2 O + formate | |
| Occurrence | ||
| Parent taxon | Vertebrates | |
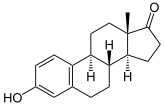
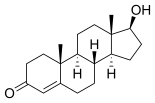
The aromatase (also CYP19A1 ) is the enzyme that in vertebrates the conversion of testosterone to estradiol or of androstenedione to estrone catalyzed . This aromatization of androgens is the crucial final step in the biosynthesis of estrogens .
Aromatase, also called estrogen synthase , is a monooxygenase ( EC 1.14.14.1 ) that uses heme as a cofactor and belongs to the cytochrome P450 family 19. The protein, therefore known as CYP19A1, is located in the membrane of the endoplasmic reticulum (ER) of cells in various tissues. It is found in the gonads , placenta , mammary gland , adipose tissue and also in the brain, as well as in skin , bones and blood vessels . Mutations in CYP19A1 - gene can be hereditary aromatase or surplus lead.
Catalyzed reactions
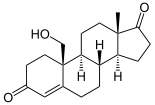
Androstenedione is flavored to estrone (E 1 ) via 19-OH-androstenedione and 19-oxo-androstenedione .
Testosterone is converted to estradiol (E 2 ) in a similar way .
The history of aromatase
Aromatase is the key enzyme in the biosynthesis of estrogens, the so-called female sex hormones. As early as the 1930s it became known that androgens (C19 steroids) can be converted into estrogens (C18 steroids). In the aromatization of the A ring of the C19 is a substituent as in the 10-position formate cleaved. Tissue extracts from porcine adrenal glands served as the source of aromatase activity in these early years of research. Before the enzyme itself could be characterized, intermediate stages of aromatization were identified, such as 19-hydroxy-androstenedione and 19-oxo-androstenedione (see figure above).
Analysis of the biochemistry and function of this enzyme was linked to the search for inhibitors of aromatase function. Because in the 1970s was not only found that aromatase outside of gonads and adrenal gland expressed is: u. a. in adipose tissue and in the mammary gland and in breast cancer cells. But also that estradiol is a growth factor of breast cancer cells, which is produced by the cancer cells and the glandular tissue itself.
An alternative way for the occurrence of estrogen or estradiol in the mammary gland tissue is the splitting off of sulfate from estrone sulfate by a sulfatase expressed in the tissue. With radioactively labeled estrone sulfate it could be shown in breast tumors of rodents that only about 20 to 50% of the estrone in the female breast was produced by the effect of sulfatase on the circulating oestrone sulfate in the blood; the remaining 80–50% therefore had to have arisen through androstenedione conversion.
The formation of new estrogens could be proven by ex vivo tests with radioactively labeled androstenedione. A radioactive H isotope ( tritium ) built into the C19 carbon of androstenedione helped to demonstrate the oxidoreductase activity of aromatase as a loss of radioactivity as a result of oxidation reactions. In a multi-step reaction process, the enzyme acts as a monooxygenase to gradually absorb three oxygen molecules at C19, with the help of NADPH cytochrome P450 oxidoreductase and NADPH (see adjacent figure).
In the 1980s, it was possible to isolate the human aromatase protein from placental microsomes . Its enzymatic effect for the conversion of androstenedione into estrogen was demonstrated on the isolated protein. This proved that the multi-step reaction is catalyzed by a single protein. The aromatase sequence was finally obtained with the aid of PCR as well as molecular biological cloning and sequencing. The corresponding gene was then localized to chromosome 15 locus q21.2.
Aromatase evolution
The formation of estrogens from androgens with the help of aromatase has long been considered a characteristic feature of vertebrates. Findings in mussels in which oestrogens have been identified and the sequencing of aromatase-like genes in other non-vertebrates have cast doubt on the vertebrate origin of aromatase. To date, however, no real aromatases in invertebrates have been genetically or functionally characterized. Tiwary and Li postulated a parallel evolution of androgen receptor and aromatase in 2009, but the invertebrate sequences used are rejected by Reitzel and Tarrant as unsuitable for the analysis.
It should also be borne in mind that estrogens are formed from cholesterol via pregnenolone, progesterone, testosterone. The conversion of cholesterol to pregnenolone by the necessary side chain- splitting enzyme CYP11A1 is vertebrate-specific. The formation of estrogens from cholesterol therefore requires a total of five enzymes. These five enzymes are present and functional in fish and then in all other vertebrates. For non-vertebrate animals, however, the chain of evidence is extremely sketchy. Sometimes there is enzyme activity, but neither protein nor gene; sometimes there is a gene but no functional protein. The emphasis is on one and not five. It therefore remains to be seen whether an invertebrate orthologue will ever be found for aromatase.
Structure and function of aromatase
Like the associated NADPH cytochrome P450 oxidoreductase (POR), aromatase is a membrane-associated protein in the endoplasmic reticulum. POR binds NADPH / H + , and activated via the FAD - and FMN - cofactors directly the active site of the aromatase, which then provides the active oxygen to attack on the C19 carbon atom of androgens available.
The aromatase gene on chromosome 15 has nine coding exons (2 to 10) and several variants for the untranslated exon 1. Under the influence of tissue-specific transcription factors , the aromatase RNA transcription is initiated at different points. The following list summarizes the aromatase gene promoter and describes the sequence of the various starting sites in the sequence from furthest away from the coding exon 2 to the closest to exon 2; the numbers in brackets indicate the distance (in kilobases ) to the next starting point:
- Placenta (major) 1.1 (15 kb)
- Placenta (minor 2) 2a (5 kb)
- Skin / fat tissue 1.4 (30 kb)
- Fetal tissue 1.5 (7 kb)
- Endothelial cells / breast carcinoma 1.7 (3 kb)
- Brain 1.f (20 kb)
- Placenta (minor 1) 1.2 (12 kb)
- Bone 1.6 (0.5 kb)
- Adipose tissue / breast cancer 1.3 (0.2 kb)
- Ovary / testes / breast cancer / endometriosis PII (0 kb)
In total, the gene spans around 120 kb (kilobase pairs). This list also shows in which human tissues the aromatase is expressed.
Function of aromatase in various tissues
In the ovaries
In many vertebrates , from fish to birds to mammals , aromatase is a special protein found in the gonads (and the brain). Human granulosa cells in pre-ovulatory (ready to jump ) follicles express the aromatase much more strongly than the cells of smaller follicles. In humans and rodents, aromatase has also been found in the corpus luteum . In the testes , aromatase is found in Sertoli cells before puberty and later in Leydig cells in adult men . Aromatase was also identified in different sperm maturation stages of rodents.
Preovulatory estradiol is the growth hormone for the uterine lining, the endometrium . Under the influence of E 2 , it prepares to take up the fertilized egg. If the corpus luteum ceases to form E 2 after ovulation and without progesterone formation from the trophoblast , the endometrium atrophies and is shed during the menstrual period .
Lessons from patients or aromatase gene knock-out mice or overexpressing mice
Patients with aromatase defects do not have clearly male or female gonads at birth, develop amenorrhea , lack of development of a female breast, hypergonadotrophic hypogonadism and cystic ovaries during puberty . Since the symptoms can be limited with estradiol administration, there are no long-term studies on this clinical picture.
Affected men (seven known cases up to 2006) lack any estradiol, but have normal levels of testosterone and gonadotropins. This is accompanied by abnormally large growth with delayed bone maturation and epiphyseal closure. In addition, there is osteoporosis with bone pain and genu valgum . Increased insulin, impaired lipid metabolism, and lack of fertility have also been described.
Mice with switched off aromatase gene (gene knock-out mice) showed u. a. Brain defects, memory disorders, autoimmunity with lymphocyte proliferation, thymus shrinkage with lower cell density, insulin resistance, obesity that increases with aging, increased cholesterol, blood lipoprotein and triglyceride values, decreasing bone length and density, decreased aggression against male disorders but increased aggressiveness against females ready to mate.
Aromatase inhibitors
Aromatase inhibitors can block the production of estrogens in muscle and fat tissue and are therefore used in the treatment of hormone-sensitive breast cancer . Since the estrogen production in the ovaries is not stopped by aromatase inhibitors, these are only suitable for women after menopause or after surgical removal or medication blockade of the ovaries. Medicinal substances used therapeutically are anastrozole , letrozole and exemestane . Aromatase inhibitors are used in breast cancer, the development and course of which is often influenced by the female sex hormone estrogen. Aromatase inhibitors are used especially in advanced breast cancer in postmenopausal women when the cancer did not respond or did not respond adequately to treatment with antiestrogens (tamoxifen).
The most common side effects of aromatase inhibitors are joint problems. These arthralgias occur in up to 50% of the treated patients, but can be alleviated somewhat with regular exercise (strength and endurance training) in order to prevent the end of therapy early. Bodybuilders also use aromatase inhibitors - especially with artificial testosterone supply - to prevent the increased conversion into estrogens due to the increased testosterone level. The World Anti-Doping Agency (WADA) lists aromatase inhibitors in the S4 category of prohibited doping substances ; Anastrozole, letrozole, aminogluthetimide, exemestane, formestane and testolactone are explicitly listed.
literature
- VJ Assikis & A. Buzdar: Recent advances in aromatase inhibitor therapy for breast cancer. In: Seminars in Oncol. 29 (3 Suppl. 11), 2002, pp. 120-128.
- Roselli CE, Liu M, Hurn PD: Brain aromatization: classic roles and new perspectives . In: Semin. Reprod. Med. . 27, No. 3, May 2009, pp. 207-17. doi : 10.1055 / s-0029-1216274 . PMID 19401952 .
Web links
- OrphaNet: Aromatase deficiency
- Jassal / reactome: Testosterone is converted to estradiol
- Jassal / reactome: Androstenedione is converted to estrone by Aromatase (CYP19A1)
Individual evidence
- ↑ Homologues at OMA
- ↑ UniProt P11511
- ↑ Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A: History of aromatase: saga of an important biological mediator and therapeutic target . In: Endocr. Rev. . 30, No. 4, June 2009, pp. 343-375. doi : 10.1210 / er.2008-0016 . PMID 19389994 .
- ↑ Basant Tiwary, Wen-Hsiung Li: Parallel evolution between aromatase and androgen receptor in the animal kingdom . In: Molecular Biology and Evolution . 26, No. 1, January 2009, pp. 123-129. doi : 10.1093 / molbev / msn233 . PMID 18936441 .
- ↑ Adam M Reitzel, Ann M Tarrant: Correlated evolution of androgen receptor and aromatase revisited . In: Molecular Biology and Evolution . May 21, 2010. doi : 10.1093 / molbev / msq129 . PMID 20494939 .
- ↑ Alan and Margaret Conley1 Hinshelwood: Mammalian aromatases . In: Reproduction . 121, 2001, pp. 685-695; PMID 11427156 .
- ^ A b Jones ME, Boon WC, Proietto J, Simpson ER .: Of mice and men: the evolving phenotype of aromatase deficiency. . In: Trends Endocrinol Metab. . 17, No. (2), 2006, pp. 55-64 .. PMID 16480891 .
- ↑ Xiangdong Li, Nafis Rahman: Impact of androgen / estrogen ratio: Lessons learned from the aromatase over-expression mice . In: General and Comparative Endocrinology . 159, 2008, pp. 1-9. PMID 18762187 .
- ↑ SABCS: Exercise Improves Drug-associated Joint Pain in Breast Cancer Survivors ( Memento of the original from December 14, 2013 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ NADA : The Prohibited List 2009 - International Standard. P. 5 ( PDF; 165 kB ).
- ^ Adams LS, Chen S: Phytochemicals for breast cancer prevention by targeting aromatase . In: Front. Biosci. . 14, 2009, pp. 3846-63. PMID 19273315 .