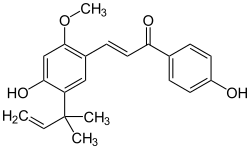
Licochalcone A
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Licochalcone A | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 21 H 22 O 4 | ||||||||||||||||||
| Brief description |
yellow, crystalline solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 338.3 g mol −1 | ||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Licochalcone A is a chemical compound called a flavonoid that can be extracted from the root of Chinese licorice ( Glycyrrhiza inflata ). In addition to the well-known anti-inflammatory effect, antibacterial, antiparasitic and cancer-inhibiting effects have so far been demonstrated in laboratory studies.
Occurrence
In licorice, Licochalcone A occurs only in the species G. inflata and G. eurycarpa , which makes it a distinguishing feature from the very similar G. glabra and G. uralensis .
Licochalcon A was also found in Indian patchouli ( Pogostemon cablin ) and Pleurospermum hookeri var. Thomsonii , an umbelliferae .
synthesis
Nothing is yet known about the biosynthesis of the licochalcones. For synthesis in the laboratory, prenylated benzaldehydes can be used as starting substances .
pharmacology
Licochalcone A inhibits the release of inflammatory mediators such as NF-κB in various skin cells . The effect against Plasmodium comes about due to an irreversible change in red blood cells. The active ingredient is used in skin care products for atopic dermatitis and in cosmetics for sensitive skin.
Individual evidence
- ↑ Data sheet Licochalcon A (PDF) from Calbiochem, accessed on December 8, 2015.
- ↑ a b Datasheet Licochalcone A from Sigma-Aldrich , accessed on April 7, 2011 ( PDF ).
- ↑ Kondo K, Shiba M, Nakamura R, Morota T, Shoyama Y: Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information . In: Biol Pharm Bull . 30, No. 7, July 2007, pp. 1271-1277. PMID 17603166 .
- ^ Rauchsteiner F, Matsumura Y, Yamamoto Y, Yamaji S, Tani T: Analysis and comparison of Radix Glycyrrhizae (licorice) from Europe and China by capillary-zone electrophoresis (CZE) . In: J Pharm Biomed Anal . 38, No. 4, July 2005, pp. 594-600. doi : 10.1016 / j.jpba.2005.01.038 . PMID 15967286 .
- ↑ Xu X, Hou C, Zhang Y, Liu Q, Liu Y, Yang J: [Chemical constituents of the roots of Glycyrrhiza eurycarpa PCLi] . In: Zhongguo Zhong Yao Za Zhi . 22, No. 11, November 1997, pp. 679-680, 703-704. PMID 11243185 .
- ↑ Park EJ, Park HR, Lee JS, Kim J: Licochalcone A: an inducer of cell differentiation and cytotoxic agent from Pogostemon cablin . In: Planta Med . 64, No. 5, June 1998, pp. 464-466. PMID 9690352 .
- ↑ Li T, Wang T: [Determination of three flavonoids and ferulic acid in Pleurospermum hookeri CB Clarke var. Thomsonii CB Clarke by HPLC] . In: Zhongguo Zhong Yao Za Zhi . 24, No. 1, January 1999, pp. 44-45, 64-inside back cover. PMID 12078155 .
- ↑ Kromann H, Larsen M, Boesen T, Schønning K, Nielsen SF: Synthesis of prenylated benzaldehyde and Their use in the synthesis of analogues of licochalcones A . In: Eur J Med Chem . 39, No. 11, November 2004, pp. 993-1000. doi : 10.1016 / j.ejmech.2004.07.004 . PMID 15501549 .
- ↑ Furusawa J, Funakoshi-Tago M, Mashino T, et al. : Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Licochalcone D, inhibit phosphorylation of NF-kappaB p65 in LPS signaling pathway . In: Int. Immunopharmacol. . 9, No. 4, April 2009, pp. 499-507. PMID 19291859 .
- ↑ Ziegler HL, Hansen HS, Staerk D, Christensen SB, Hägerstrand H, Jaroszewski JW: The antiparasitic compound licochalcone a is a potent echinocytogenic agent that modifies the erythrocyte membrane in the concentration range where antiplasmodial activity is observed . In: Antimicrob Agents Chemother . 48, No. 10, October 2004, pp. 4067-4071. doi : 10.1128 / AAC.48.10.4067-4071.2004 . PMID 15388483 . PMC 521868 (free full text).
