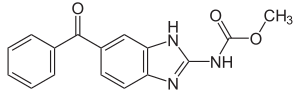
Mebendazole
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Mebendazole | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 16 H 13 N 3 O 3 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 295.29 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
288-289 ° C |
||||||||||||||||||
| solubility |
very bad in water (71.3 mg l −1 at 25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Mebendazole (trade name u a Vermax.. ® ; initial manufacturer Janssen Pharmaceutica ) is a drug selected from the group of benzimidazoles, as the worm agent in the treatment of worm diseases is used.
Clinical information
Mebendazole preparations are approved for the treatment of patients with intestinal worm infections by nematodes and some tapeworms (tablets with 100 mg) as well as cystic echinococcosis , alveolar echinococcosis and trichinosis (tablets with 500 mg). Mebendazole is also used in veterinary medicine for a variety of worm diseases.
The agents are contraindicated in the event of hypersensitivity to one of the ingredients. Concomitant administration with metronidazole , phenytoin or carbamazepine should be avoided. Mebendazole is teratogenic in rodents ; use during pregnancy is usually out of the question.
The most common side effects are abdominal pain, diarrhea, gas, nausea and vomiting, as well as headache and dizziness.
Pharmacological properties
Mebendazole binds to the microtubules in the intestine of the worms, which leads to degeneration and interruption of glucose uptake there. Mammalian cells are not affected. The absorption of mebendazole after oral administration is incomplete. A strong first-pass effect also eliminates a large part of the active ingredient, so that only a small part of the dose is bioavailable. The drug was developed by Janssen Pharmaceutika in the early 1970s.
research
According to a recent study, mebendazole has cytostatic effects and acts against gliomas, i.e. brain tumors.
Trade names
Pantelmin (A), Surfont (D), Vermox (D, CH). In veterinary medicine it is marketed under the names Mebentab and Telmin Paste , as a combination preparation with Closantel (Closantel sodium 2H 2 O) as Flukiver Combi .
Web links
- Entry on mebendazole at Vetpharm, accessed on August 11, 2012.
Individual evidence
- ↑ a b c Entry on mebendazole in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Mebendazole data sheet from Sigma-Aldrich , accessed on November 4, 2016 ( PDF ).
- ↑ technical information Vermox.
- ^ Michelle De Witt, Alexander Gamble, Derek Hanson, Daniel Markowitz, Caitlin Powell: Repurposing Mebendazole as a Replacement for Vincristine for the Treatment of Brain Tumors . In: Molecular Medicine (Cambridge, Mass.) . tape 23 , April 5, 2017, doi : 10.2119 / molmed.2017.00011 , PMID 28386621 .