Meldola blue
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Meldola blue | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| Brief description |
dark green to black crystal powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | |||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
280 ° C with decomposition |
||||||||||||||||||
| solubility |
soluble in water and in ethanol |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
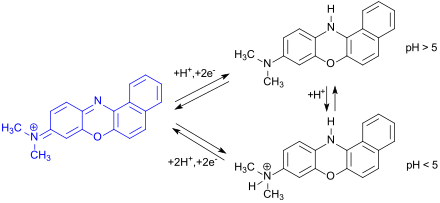
Meldola Blue is a blue phenoxazine - dye whose synthesis and properties for the first time in 1879 by Raphael Meldola described.
The basic (cationic) 1,4-oxazine derivative is in the form of a chloride salt; in most cases, however, the better crystallizing hemi - zinc chloride compound (compare, for example, brilliant cresyl blue ) is isolated.
Occurrence and representation
According to the original procedure , the N , N -dimethyl-4- nitrosoaniline formed in the nitrosation of N , N -dimethylaniline is isolated as the hydrochloride and then added to a solution of 2-naphthol in glacial acetic acid at 110.degree .
When it cools down, "magnificent, long, bronze-colored needles with a copper sheen" form.
An industrial process avoids the isolation of the toxic nitroso compound and describes the addition of a suspension of the intermediate stage in an aqueous-alcoholic medium to an ethanolic β-naphthol solution at 70 ° C, which contains anhydrous zinc chloride. When it cools down, the double salt of Meldola Blau precipitates with zinc chloride.
properties
As a double salt with zinc chloride, Meldola blue is a dark green to black crystalline solid that dissolves in water in blue-violet and in ethanol in dark blue color with an absorption maximum λ max at 650 nm (in 50% aqueous ethanol). In polar solvents, such as. For example, pyridine , ethylene glycol and cellosolve , Meldola blue is also readily soluble.
Applications
Meldola blue was initially thought to be a useful blue textile dye; However, because of its low absorption by wool and silk fibers, it could not hold its own against the much more light-stable indigo . Its technical applications are limited to the coloring of paper and leather.
Meldola blue has gained greater importance as a redox-active dye in biology. The color change from blue to the colorless leuco form during electron acceptance ( reduction ) can be used to determine reducing coenzymes , such as B. NADH or NADPH and the corresponding dehydrogenases in aqueous solutions, but also on carriers (paper strips) or in biosensors .
Individual evidence
- ↑ Entry on BASIC BLUE 6 in the CosIng database of the EU Commission, accessed on February 6, 2020.
- ↑ a b Meldola's blue. (PDF; 186 kB) Carbosynth Ltd., March 22, 2019, accessed on February 5, 2020 (English).
- ↑ a b c R. Meldola: Effect of nitrosodimethylaniline on phenols which do not contain the methyl group . In: Ber. German Chem. Ges. Volume 12 , no. 2 , 1879, p. 2065-2066 , doi : 10.1002 / cber.187901202221 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ R. Meldola: V. On a new class of coloring matters from the phenols . In: J. Chem. Soc. tape 39 , 1881, p. 37-40 , doi : 10.1039 / CT8813900037 .
- ↑ Patent US3665601 : Process for the manufacture of basic oxazine dyestuffs. Applied on February 13, 1969 , published on April 11, 1972 , applicant: Farbwerke Hoechst AG, inventor: N. Ottawa, G. Schäfer.
- ^ A b Edward Gurr: Synthetic Dyes in Biology, Medicine and Chemistry . Academic Press, London, UK 1971, ISBN 978-0-12-309650-0 , pp. 74-75 .
- ↑ M. Hope Roberts, RW Horobin: Biomedical applications and chemical nature of three dyes first Synthesized by Raphael Meldola: isamine blue, Meloda's blue and naphthol green B . In: Biotechnic & Histochemistry . tape 8 , no. 4 , 2012, p. 295-299 , doi : 10.3109 / 10520295.2011.639722 .
- ↑ P. Kugler, K.-H. Wrobel: Meldola Blue: A new electron carrier for the histochemical demonstration of dehydrogenases . In: Histochemistry . tape 59 , no. 2 , 1978, p. 97-109 , doi : 10.1007 / bf00518505 .
- ↑ Patent DE1959410 : Indicator for determining the reduced pyridine coenzymes . Registered on November 26, 1969 , published on June 16, 1971 , applicant: Boehringer Mannheim GmbH, inventors: HU Bergmeyer, E. Haid, M. Nelboeck-Hochstetter, G. Weimann.