Membrane fusion
The membrane fusion (neurophysiology) refers to a biochemical process in neuronal cells, wherein the synaptic vesicle which the excitation forwarding are filled with neurotransmitters via exocytosis pinched off at the presynaptic membrane and the neurotransmitter molecules (eg. As acetylcholine, γ-aminobutyric acid) in The synaptic gap are released, from where they then pass the signal on to the downstream target cell (e.g. gland cells, muscle cells, nerve cells ).
Basics
Cytoskeleton
→ Main article: cytoskeleton
The cytoskeleton is a network of numerous proteins that line the intracellular space of all eukaryotic cells and are responsible for some metabolic and transport processes within the cell. The cell division , u. a. consisting of mitosis and cytokinesis, is induced by certain protein fibers of the cytoskeleton.
The proteins that make up the cytoskeleton can be divided into five different categories: bridging proteins , limiting proteins , regulating proteins , motor proteins and scaffolding proteins . The latter two play a fundamental role in membrane fusion.
The protein framework of the cell is made up of several components. On the one hand by the of tubulin existing microtubule and on the other by those from actin existing microfilaments . Microtubules and microfilaments of a cell are summarized as microfibrils. However, since membrane fusion relates to nerve cells , we speak of neurotubules and neurofilaments, which are grouped together as neurofibrils. As scaffold proteins , they belong to the class of cytoskeletal proteins .
While the actin-made neurofilaments (approx. 10 nm wide) primarily support the strength of the cell, the neurotubule (approx. 25 nm wide and hollow inside) already has other tasks, such as axonal transport both in anterograde and in retrograde direction.
Organization of the microtubule
Microtubules are tubular protein fibers that are part of the cytoskeleton. Two basic tubulin molecules make up the microtubule and create negative and positive charges on the surface of the microtubule. The α-tubulin with its negative charge takes one end (see minus end) and the β-tubulin with its positive charge the other end (see plus end) of a so-called protofilament. Several of these protofilaments then come together to form microtubules .
The microtubule has a specific direction, caused by the anchoring of the negative pole in the microtubule organizing center (MTOC for microtubule organizing center ), which can be formed by centrioles , for example . However, since there are no centrioles in adult nerve cells, which results in the loss of the ability to divide , the MTOC is organized differently in this cell type. The positive pole ends in nerve cells further distal, i.e. away from the cell body ( perikaryon ), runs through the axon and finally opens into the synaptic end bulb.
Axonal transport
→ Main article: Axonal transport
In the following, axonal transport is related to synaptic vesicles, which are synthesized in the cell body, the perikaryion. The transmitter molecules are then packaged by the Golgi apparatus so that the vesicles are now ready for transport into the synaptic end bulb. This neurotubule-dependent transport is also summarized as fast axonal transport (50–400 mm per day), from which the slow axonal transport (0.2–5 mm per day) is distinguished. However, the slow axonal transport plays a negligible role in membrane fusion.
Depending on the particular motor protein , the neurotubule-dependent transport takes place either in an anterograde (to the synapse) or in a retrograde (to the perikaryon) direction. The motor proteins responsible for this, which form a category of cytoskeletal proteins, include kinesin , dynein and myosin . These motor proteins all have a typical structure. The motor domain docks to the neurotubules and ensures the locomotion along the protein fibers, while the tail domain binds the synaptic vesicles, containing transmitter molecules, and pulls them with it. The movement is based on the various charges of the tubulin molecules. Since dynein is responsible for retrograde transport, kinesin and myosin are suitable for axonal transport of the vesicles into the synaptic end bulb. Still, there is a difference: kinesin actually binds to the neurotubule and transports vesicles and cell organelles, while myosin binds to the actin filaments of the cytoskeleton, but also transports vesicles and is able to move them and induce exocytosis (and other contractions) .
After the vesicles have formed on the Golgi apparatus, they bind to the motor proteins kinesin and myosin, which the vesicles then transport into the synaptic end bulb . Once there, the vesicles detach themselves from the motor proteins and bind to other protein fibers in the end bulb via other membrane proteins, so that the synaptic vesicles are available for membrane fusion.
Synaptic vesicles

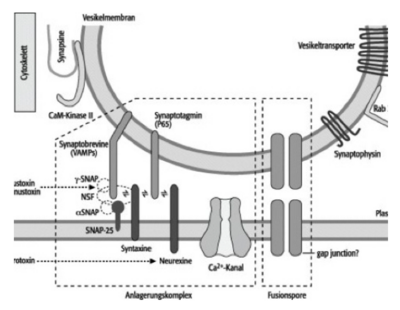
Synaptic vesicles, i.e. those vesicles formed by the Golgi apparatus that contain transmitter molecules for signal transduction at the synapse , have a typical fine structure. So-called SNARE proteins (English abbreviation: soluble N-ethylmaleimide-sensitive-factor attachment receptor) play a fundamental role. There are numerous membrane proteins such as synaptobrevins , synaptotagmins and synaptophysins on the membrane of the vesicle . They catalyze the transport of the vesicles and exocytosis on the presynaptic membrane.
But first the vesicles are connected to the protein fibers of the cytoskeleton via synapsins, thin membrane proteins, which run through the synaptic end bulb. These synapsins ensure that the vesicles do not arbitrarily constrict through exocytosis and release neurotransmitters .
The SNARE proteins have the typical structure of a peptide. The N-terminal with the amino group ( ) is positively charged in the body through the addition of an H atom of the carboxyl group of the C-terminal ( ), while the carboxyl group ( ) at the C-terminal splits off an H atom and consequently a negative charge accepts ( ). The negative and positive partial charges later play an important role in membrane fusion (see zwitterion ).
Membrane fusion
If an action potential reaches the synaptic end bulb as a result of the conduction of excitation via the axon, neurotransmitters are released into the synaptic gap, which exhibit an excitatory or inhibitory effect on the target cell (effector). The release of the transmitter molecules or the constriction of the vesicles on the presynaptic membrane is induced by an action potential, which opens voltage-dependent channels, which results in an influx of calcium ions. The increasing concentration of calcium ions activates certain enzymes, including the calmodulin-dependent protein kinase II . This enzyme phosphorylates the synapsins that connect the synaptic vesicles to the protein fibers of the cytoskeleton. The phosphorylation loosens precisely these bonds and the vesicles completely detach from the protein fibers.
As already described above, the motor protein myosin is able to transport vesicles anterograde and induce exocytosis. By releasing the vesicles from the protein fibers, they are now available for further transport towards the presynaptic membrane. Bound to the tail domain of the myosin, the vesicles are transported on until they are finally pinched off via the exocytosis.
Certain membrane proteins, so-called neurexins and syntaxins, are also found on the presynaptic membrane . Due to the above-mentioned different partial charges of the proteins at the N and C terminals, the membrane proteins of the vesicles interact with the receptor proteins of the presynaptic membrane. SNARE complexes accumulate and exocytosis can take place: the vesicles are pinched off and the transmitters are released.
Effects of neurotoxins
Certain nerve toxins are able to intervene in the signal transmission of the nerve cells and hinder membrane fusion. One of the best known neurotoxins capable of this is botulinum toxin (BTX), which comprises several neurotoxic proteins secreted by strains of the bacterium Clostridium botulinum . In the human body, it prevents the release of transmitter molecules. The consequences are respiratory paralysis and cardiac arrest. The constriction of the vesicles is prevented by the fact that certain types of BTX, including types B, D, F and G, break down protein complexes on the vesicle membrane, including the SNARE protein synaptobrevin. Type C, on the other hand, destroys the receptor protein syntaxin. Since these proteins form the basis of the membrane fusion, it can no longer take place.
See also
literature
- Martin Trepel: Neuroanatomy - Structure and Function. 6th edition. Elsevier / Urban & Fischer, Munich 2015, ISBN 978-3-437-41287-5 .
- Michael Schuler, Werner Waldmann (ed.): Health Altlas Anatomy. The human body and its functions in over 600 illustrations. Naumann & Göbel Verlag, Cologne 2009, ISBN 978-3-625-12654-6 .
Web links
- Anatomy and Physiology by VisibleBody
- Atlas of Human Anatomy by VisibleBody
- Modulation of the signal transmission from the Max Planck Society
Individual evidence
- ↑ Neurotubules - Lexicon of Biology . ( Spektrum.de [accessed on October 20, 2017]).
- ↑ synaptic vesicles - Lexicon of Biology . ( Spektrum.de [accessed on October 20, 2017]).
- ↑ SNARE - Lexicon of Biology . ( Spektrum.de [accessed on October 20, 2017]).
- ↑ Synapsine - Encyclopedia of Neuroscience . ( Spektrum.de [accessed on October 20, 2017]).
- ↑ Calcium-Calmodulin-Dependent Protein Kinase II - Lexicon of Neuroscience . ( Spektrum.de [accessed on October 20, 2017]).
- ^ Membrane fusion - Lexicon of Biology . ( Spektrum.de [accessed on October 20, 2017]).
- ^ DocCheck Medical Services GmbH: Botulinum toxin - DocCheck Flexikon. Retrieved October 20, 2017 .