Mesotrione
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
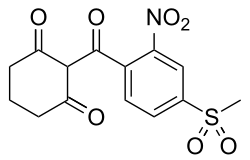
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Mesotrione | |||||||||||||||
| other names |
2 - [(4-methylsulfonyl) -2-nitrobenzoyl] cyclohexane-1,3-dione |
|||||||||||||||
| Molecular formula | C 14 H 13 NO 7 S | |||||||||||||||
| Brief description |
yellowish solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 339.32 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.49 g cm −3 (bulk density) |
|||||||||||||||
| Melting point |
165.3 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Mesotrione is an active ingredient for crop protection and a chemical compound from the group of cyclohexane derivatives .
history
Mesotrione is a herbicide -Wirkstoff from the class of Triketones which on leptospermone from Callistemon citrinus decline. Its herbicidal effect was found in 1977 by a researcher at the Western Research Center of Stauffer Chemical .
Extraction and presentation
Mesotrione can be obtained by reacting 1,3-cyclohexanedione with 2-nitro-4-methylsulfonylbenzoyl chloride.
properties
Mesotrione is a yellowish solid.
use
Mesotrione works by inhibiting 4-hydroxyphenylpyruvate dioxygenase (HPPD), an important enzyme in the biosynthesis of carotenoids . It was brought onto the market in 2001. Nitisinone is a derivative of mesotrione , and sulcotrione is structurally similar .
Admission
The active ingredient mesotrione was approved for use as a herbicide in the European Union in 2003.
In Germany, Austria and Switzerland, plant protection products with this active ingredient are approved. The most important area of application is corn cultivation.
Individual evidence
- ↑ a b c Entry on mesotrione in the GESTIS substance database of the IFA , accessed on January 29, 2012(JavaScript required) .
- ↑ a b Entry on mesotrione in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on August 1, 2013.
- ↑ Entry on 2- [4- (methylsulfonyl) -2-nitrobenzoyl] -1,3-cyclohexanedione in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can use the harmonized classification and labeling expand .
- ↑ Mesotrione data sheet at Sigma-Aldrich , accessed on May 20, 2017 ( PDF ).
- ↑ a b History of mesotrione
- ↑ Renaud Beaudegnies, Andrew JF Edmunds, Torquil EM Fraser, Roger G. Hall, Timothy R. Hawkes, Glynn Mitchell, Juergen Schaetzer, Sebastian Wendeborn, Jane Wibley: Herbicidal 4-hydroxyphenylpyruvate dioxygenase inhibitors – A review of the triketone chemistry story from a Syngenta perspective . In: Bioorganic & Medicinal Chemistry . tape 17 , no. June 12 , 2009, p. 4134-4152 , doi : 10.1016 / j.bmc.2009.03.015 ( PDF ).
- ↑ Raj B. Durairaj: resorcinol: chemistry, technology, and applications . Springer, Berlin / Heidelberg 2005, ISBN 3-540-25142-1 .
- ↑ Josie Annalee Hugie: Understanding the interaction of mesotrione and atrazine in redroot pigweed . University of Illinois at Urbana-Champaign ( p. 4 in Google Book Search).
- ↑ Directive 2003/68 / EC of the Commission of July 11, 2003 amending Council Directive 91/414 / EEC to include the active substances trifloxystrobin, carfentrazone-ethyl, mesotrione, fenamidone and isoxaflutole (PDF)
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on mesotrione in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 12, 2016.
