Methyl benzoyl formate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
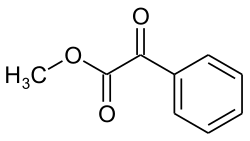
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Methyl benzoyl formate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 8 O 3 | |||||||||||||||
| Brief description |
yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 164.16 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.155 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
16 ° C |
|||||||||||||||
| boiling point |
246-248 ° C |
|||||||||||||||
| Vapor pressure |
<1 hPa (20 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| Refractive index |
1.526 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Methylbenzoyl formate is a chemical compound from the group of formates and benzoyles .
Extraction and presentation
Methylbenzoyl formate can be obtained by reacting phenylglyoxylic acid with methanol .
properties
Methyl benzoyl formate is a yellow liquid that is practically insoluble in water.
use
Methyl benzoyl formate is used as a photoinitiator .
Individual evidence
- ↑ a b c d e f Entry on methylbenzoyl formate in the GESTIS substance database of the IFA , accessed on April 4, 2020(JavaScript required) .
- ↑ a b c d e f data sheet methyl benzoylformate, 98% from Sigma-Aldrich , accessed on April 3, 2020 ( PDF ).
- ↑ Google Patents: CN105330547A - Methyl benzoylformate highly selective synthetic method - Google Patents , accessed April 4, 2020
- ^ GWA Milne: Gardner's Commercially Important Chemicals Synonyms, Trade Names, and Properties . John Wiley & Sons, 2005, ISBN 0-471-73661-9 , pp. 401 ( limited preview in Google Book search).