Midodrine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Midodrine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 12 H 18 N 2 O 4 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
α 1 adrenoceptor agonist |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 254.28 g mol −1 | |||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Midodrin ( trade name Gutron ® ) is a drug used to treat hypotonic circulatory disorders ( orthostatic hypotension ). It is a prodrug whose metabolite desglymidodrin is the actual active ingredient.
The drug is used in the form of its hydrochloride .
Chemical structure
Midodrin contains the basic structure 2-phenylethylamine , which is also the basis of the body's own neurotransmitters adrenaline and noradrenaline .
Stereochemistry
Midodrine contains a stereocenter and consists of two enantiomers. This is a racemate , i.e. a 1: 1 mixture of ( R ) - and ( S ) -form:
| Enantiomers of midodrine | |
|---|---|
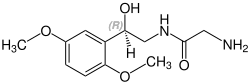 CAS number: 133163-25-4 |
 CAS number: 133267-39-7 |
Pharmacokinetics and Effect
By amide cleavage ( deglycination ), the active substance desglymidodrine is produced in the organism from the precursor midodrine . This can only cross the blood-brain barrier with difficulty. Desglymidodrine is an agonist at α 1 -adrenoceptors (direct sympathomimetic ) and causes an increase in the tone of the arterial and venous vascular muscles and thereby increases blood pressure ( vasoconstriction ).
Trade names
Gutron (D, A, CH)
Individual evidence
- ↑ a b c Entry on midodrin. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ a b Datasheet Midodrine hydrochloride from Sigma-Aldrich , accessed on October 16, 2016 ( PDF ).
- ↑ Red List online, as of October 12, 2017.
- ↑ AM comp. d. Switzerland, as of October 2009.
- ↑ AGES-PharmMed, as of October 2009.
- ↑ HJ Roth u. H. Fenner: Drugs . Thieme, Stuttgart a. New York 1988, pp. 408-409.
- ↑ Rote Liste Service GmbH (Ed.): Rote Liste 2017 - drug directory for Germany (including EU approvals and certain medical devices) . Rote Liste Service GmbH, Frankfurt / Main, 2017, edition 57, ISBN 978-3-946057-10-9 , p. 196.
