Sodium cocoyl isethionate
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| General | |||||||||||||
| Surname | Sodium cocoyl isethionate | ||||||||||||
| other names |
Coconut fatty acid 2-sulfoethyl ester, sodium salt |
||||||||||||
| Molecular formula | C 14 H 27 O 5 NaS | ||||||||||||
| Brief description |
colorless solid as a free-flowing powder or grain |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | approx. 330 g mol −1 as C 12 carboxylic acid ester | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
293.07 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
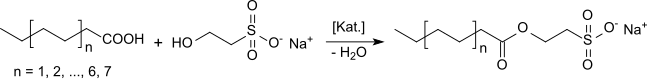
Sodium cocoyl isethionate is an ester of long-chain aliphatic carboxylic acids ( fatty acids ) obtained from coconut oil with isethionic acid or sodium isethionate and belongs to the class of isethionates, which are also referred to in the literature as acyl isethionates or, according to chemical nomenclature, as 2-sulfoethyl carboxylic acid esters or acyloxyethane sulfonates. (The expanded definition according to: “ Isethionates are anionic surfactants with an –O – CH 2 CH 2 –SO 3 - group ” also includes ethers with long-chain alcohols - in the specific case alkyl or alkenyl oligoglycosides.) The most important representative This class of mild anionic surfactants is sodium cocoyl isethionate, which is referred to in the English literature as sodium cocoyl isethionate (SCI). As a large-volume chemical product, SCI was examined in the " High Production Volume (HPV) Chemical Challenge Program" of the US Department of the Environment (EPA).
Like Tauride, isethionates were commercialized in the late 1920s under the brand name Igepon R (Igepon A = sodium cocoyl isethionate, Igepon T = N-methyl-N-oleyl taurate) as particularly mild detergent substances that are resistant to hard water.
Presentation and extraction
Isethionates can be produced via the so-called "indirect route" by reacting higher carboxylic acid chlorides with sodium isethionate . In industrial processes, SCI is obtained by direct esterification of a coconut fatty acid mixture with sodium isethionate in the presence of catalysts.
For this purpose, a coconut fatty acid mixture of different alkyl chain lengths Cn, typically C6 = 0.4% by weight; C8 = 7.6; C10 = 6.5; C12 = 47.7; C14 = 18.4; C16 = 8.9; C18 = 6.2; C18: 1 = 3.7 in excess with sodium isethionate solution and zinc oxide under nitrogen at approx. 200 ° C for reaction. After the water has been distilled off from the sodium isethionate solution and the water of reaction, a viscous mass is formed which is liquefied again by adding paraffin wax. After esterification with conversions> 95%, stearic acid is added in order to lower the solidification point of the mixture below 50.degree. When using branched fatty acids, there is no need to add paraffin as a consistency regulator and you get highly concentrated acyloxyethanesulfonates with high foaming power, good hard water stability and also good water solubility (up to 30% at 20 ° C).
properties
Solid SCI is available in flake, granulate or powder form with approx. 85% active content (SCI-85). There are also solid SCI-65 flakes with around 30% fatty acid content. SCI is not very soluble in water and is not long-term stable in solution because of its ester bond in the molecule at pH values below 5 and above 8. Sodium cocoyl isethionate is non-toxic in animal experiments and has little toxicity to aquatic organisms. It is easily biodegradable with a low affinity for bioaccumulation. SCI is slightly to moderately irritating to the skin and eyes. SCI exposure can cause minimal to mild skin irritation, although it does not cause skin sensitization.
use
Because of its excellent skin compatibility , its pronounced foam formation and foam stability, SCI is also used in hard water, its good cleaning effect and its pleasant skin feel in bars of soap, the so-called syndet bars or in combination with soaps in the so-called combo bars, which are used in particular as baby soaps Find. Because of its low water solubility (approx. 0.01% = 100ppm at 25 ° C), SCI has to be solubilized for use in liquid washing solutions, i.e. H. its concentration in the soap micelles can be increased. This z. B. the addition of secondary surfactants or the exchange of sodium for ammonium cations [ammonium cocoyl isethionate is very soluble in water with> 25 wt.% At 25 ° C].
A clear, liquid and inexpensive washing solution with 10% SCI has the following composition:
| INCI name | Weight% |
|---|---|
| Ammonium lauryl sulfate | 7.00 |
| Ammonium laureth sulfate | 5.25 |
| Cocamide MEA | 1.75 |
| Sodium cocoyl isethionate | 10.00 |
| Preservatives | q. s. |
| Fragrance | q. s. |
| De-ionized water | ad 100% |
In addition to bar soap, sodium cocoyl isethionate is used in shampoos, shower gels, shaving foam, washing lotions and other cosmetic preparations.
Individual evidence
- ↑ a b Jordapon R Grades, Technical Information, April 2008, BASF SE.
- ↑ a b c d Assessment Plan for Fatty acids, coco, 2-sulfoethylesters, sodium salts (Sodium Cocoyl Isethionate; CAS # 61789-32-0) in Accordance with the USEPA High Production Volume Chemical Challenge Program, Prepared for: The Sodium Ethyl Sulfonates Coalition, November 24, 2006 and IUCLID Data Set, Substance ID: ACI, date: 24-NOV-2006.
- ↑ Entry on fatty acids, coconut and 2-sulfoethyl esters, sodium salts in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Patent WO9309125 : Alkyl and / or Alkenyl Oligoglycoside Isethionates. Published on May 13, 1993 , applicant: Henkel KGaA, inventor: Manfred Weuthen.
- ↑ A. Barel et al .: Handbook of Cosmetic Science and Technology. Marcel Dekker, New York / Basel 2001, ISBN 0-203-90381-1 , p. 436.
- ^ Stephan H. Lindner: Hoechst - An IG Farben plant in the Third Reich. 2nd Edition. Verlag CH Beck, Munich 2005, ISBN 3-406-52959-3 .
- ↑ M. Friedman: Chapter 5: Chemistry, Formulation, and Performance of Syndet and Combo Bars . In: L. Spitz (Ed.): SODEOPEC Soaps, Detergents, Oleochemicals, and Personal Care Products. AOCS Press, Champaign, IL 2004.
- ↑ Patent US3320292 : Preparation of sulfonated fatty acid ester surface-active agents. Published on May 16, 1967 , Applicant: Lever Brothers Ltd., Inventor: Arno Cahn, Henry Lemaire, Vincent Lamberti, Robert A. Haass.
- ↑ Patent EP0585071 : Process for making sodium acylisethionates. Published on March 2, 1994 , Applicant: Hoechst Celanese Corp., Inventor: James F. Day, Wolf-Dieter Müller, Rainer H. Muth.
- ↑ Patent EP0735021 : Process for the preparation of acyloxyalkanesulfonates. Published on October 2, 1996 , applicant: Hoechst AG, inventor: Dirk Buehring.
- ↑ Specification Hostapon 85 C from Essential Ingredients, accessed on March 6, 2017 (PDF).
- ↑ Specification Hostapon 65 C from Essential Ingredients, accessed on March 6, 2017 (PDF).
- ↑ Material Safety Data Sheet from Clariant Corp., MATERIAL SAFETY DATA SHEET Hostapon SCI-85 P
- ↑ a b J.Z. Sun et al., Solubilization of sodium cocoyl isethionate. In: Journal of Cosmetic Science 54, November / December 2003, pp. 559-568, PMID 14730372 .