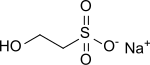
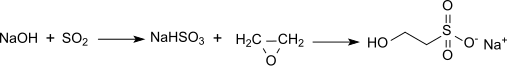Sodium isethionate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Sodium isethionate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 2 H 5 NaO 4 S | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 148.11 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
800 kg m −3 ( bulk density ) |
||||||||||||||||||
| Melting point |
192-194 ° C |
||||||||||||||||||
| solubility |
650 g l −1 at 20 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Sodium isethionate also English sodium isethionate (SI) called, which is sodium salt of 2-hydroxyethanesulfonic acid ( isethionic acid ) and is suitable because of its distinctive polarity and resistance to multivalent ions as a hydrophilic head group in washing-active surfactants , known as isethionates (Acyloxyethansulfonaten). As a large-volume chemical product, sodium isethionate was examined in the " High Production Volume (HPV) Chemical Challenge Program" of the US Department of the Environment (EPA).
Extraction and presentation
Sodium isethionate is formed when ethylene oxide reacts with sodium hydrogen sulfite in an aqueous solution:
In order to avoid impurities and to suppress the formation of (difficult to remove) by-products, the reaction must be carried out with careful control of the mass ratios and process conditions. Excess sulfite (SO 3 2− ) or bisulfite (HSO 3 - ) would lead to unpleasant smelling by-products, higher proportions of ethylene glycol or glycol ethers result in hygroscopic and greasy surfactants. Ethylene glycol-containing concentrated SI solutions can subsequently be extracted by continuous extraction with z. B. Isopropanol can be freed from ethylene glycol as far as possible (<0.5%). Therefore, in continuous industrial processes with exact control of the stoichiometry of the reactants, the temperature, the pH and the throughput with exclusion of oxygen in a z. B. designed as a packed column first reactor made of sodium hydroxide and sulfur dioxide an aqueous sodium hydrogen sulfite solution, which is mixed with a small excess of ethylene oxide and converted in a second tubular reactor at elevated temperature and pressure with precise control of the pH value practically quantitatively to sodium isethionate.
properties
As a result of the process, the aqueous solution obtained has a weakly alkaline pH value of approx. 10 and a concentration of 43% by weight sodium isethionate and contains less than 0.5% by weight ethylene glycol. A 53% strength by weight solution is usually used to produce the isethionates. The solid SI is a colorless, free-flowing, non-hygroscopic solid that dissolves very well in water and is readily biodegradable. Because of its excellent skin tolerance, SI is added to soaps and liquid skin cleansing agents in up to 15 parts by weight.
use
The addition of sodium isethionate to electroplating baths allows higher current densities and lower concentrations than the much more expensive methanesulfonic acid with an improved external appearance.
The so-called biological buffer substances, such as z. B. HEPES , MES , PIPES etc. easily accessible.
By far the most important use of sodium isethionate is in the production of isethionates. These are particularly mild and well-foaming surfactants that are suitable for cleaning sensitive skin and are therefore mainly used in baby soaps and shampoos.
Individual evidence
- ↑ a b c d Entry for CAS no. 1562-00-1 in the GESTIS substance database of the IFA , accessed on August 15, 2012(JavaScript required) .
- ↑ a b Data sheet 2-Hydroxyethanesulfonic acid sodium salt (PDF) from Merck , accessed on August 15, 2012.
- ↑ a b Entry on Sodium Isethionate at TCI Europe, accessed on August 15, 2012.
- ↑ Assessment Plan for Ethanesulfonic Acid, 2-Hydroxy-, Monosodium Salt (Sodium Isethionate, CAS # 1562-00-1) in Accordance with the US EPA High Production Volume Chemical Challenge Program (PDF; 292 kB).
- ↑ U.S. Patent US 2,810,747; Inventor: AR Sexton, EC Britton; Applicant: The Dow Chemical Co., issued October 22, 1957
- ↑ U.S. Patent US 4,003,925; Inventor: V. Lamberti, BA DiLorenzo; Applicant: Lever Brothers Co., issued January 18, 1977.
- ↑ Data sheet from Oriental Union Chemical Co., Sodium Isethionate - An Eco-friendly Surfactant for Industrial Applications , (PDF; 90 kB) , accessed on August 17, 2012.
- ↑ US patent US 6,183,619 B1; Inventor: HD Gillman et al .; Applicant: Technic Inc., Specialty Chemical Systems Inc .; issued on February 6, 2001.
- ↑ US patent application US 2006/0089509 A1; Inventor: GT Carroll et al .; Applicant: Arkema Inc .; published April 27, 2006.
- ↑ Cosmetics and Hygiene , edited by W. Umbach, 3rd completely revised and expanded edition, Verlag Wiley-VCH, 2004, ISBN 978-3-527-30996-2 .