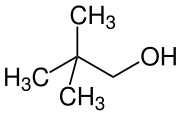
2,2-dimethyl-1-propanol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,2-dimethyl-1-propanol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 12 O | |||||||||||||||
| Brief description |
white crystalline powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 88.15 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.81 g cm −3 |
|||||||||||||||
| Melting point |
53 ° C |
|||||||||||||||
| boiling point |
113 ° C-114 ° C |
|||||||||||||||
| solubility |
40 g / l (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2,2-Dimethyl-1-propanol (also neo- pentanol) is an organic chemical compound from the group of alcohols . It is one of eight structural isomers of pentanols . It is the only alcohol from the pentanol group that is solid at room temperature.
Extraction and presentation
2,2-Dimethyl-1-propanol is obtained by reducing pivalic acid. It can also be produced from diisobutylene via the intermediate step of the peroxide . It can also be obtained via hydroformylation of isobutylene and subsequent hydrogenation .
properties
2,2-Dimethyl-1-propanol forms colorless crystals with a peppermint-like odor.
use
2,2-Dimethyl-1-propanol is used as the solvent. It serves as a ligand for transition complexes and polymerization catalysts and in organic synthesis as a reagent for introducing a neopentyl group into organic compounds.
Individual evidence
- ↑ a b c d e f g Entry on 2,2-dimethyl-1-propanol in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b c d e f Entry on pentanols. In: Römpp Online . Georg Thieme Verlag, accessed on May 15, 2018.
- ↑ Joseph Hoffman: neopentyl ALCOHOL In: Organic Synthesis . 40, 1960, p. 76, doi : 10.15227 / orgsyn.040.0076 ; Coll. Vol. 5, 1973, p. 818 ( PDF ).
- ↑ Irving Wender, Julian Feldman, Sol Metlin, Bernard H. Gwynn, Milton Orchin: Formation of Neopentyl Alcohol from Isobutylene in the Hydroformylation Reaction . In: Journal of the American Chemical Society . tape 77 , no. 21 , 1955, pp. 5760-5761 , doi : 10.1021 / ja01626a102 .

