Nitrosyl sulfuric acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
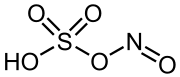
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Nitrosyl sulfuric acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | NOHSO 4 | |||||||||||||||
| Brief description |
colorless pungent smelling crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 127.08 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
Decomposes at 73.5 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Nitrosyl sulfuric acid is a chemical compound with the formula (NO) HSO 4 . The salt is a source of the nitrosyl ion (NO + ) and belongs to the group of hydrogen sulfates .
Occurrence
Nitrosylsulfuric acid is produced, for example, when cleaning exhaust gases ( flue gas ) from the metalworking industry when wet cleaning processes with sulfuric acid are used.
Extraction and presentation
Nitrosylsulfuric acid is formed when nitrogen oxides react with concentrated sulfuric acid (e.g. as a by-product in the lead chamber process ). It can also be obtained by reacting nitric acid with sulfuric acid or by introducing sulfur dioxide into nitric acid.
use
Nitrosyl sulfuric acid is used:
- for the production of caprolactam , dyes and pesticides
- for the diazotization of amines (as a substitute for nitrosylsulfuric acid, other NO + -relating compounds such as nitrosyl tetrafluoroborate [NO] BF 4 or nitrosyl chloride can be used).
Individual evidence
- ↑ a b c d Entry on nitrosylsulphuric acid in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ G. Brauer (Ed.), Handbook of Preparative Inorganic Chemistry 2nd ed., Vol. 1, Academic Press 1963, p. 406.



