Nomegestrol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
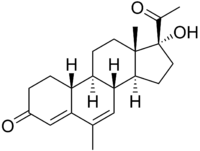
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Nomegestrol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula |
|
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 370.48 g · mol -1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Nomegestrol is a synthetically produced gestagen ( progestin ). Its ester nomegestrol acetate is used as a medicinal substance in combination with estradiol for contraception ( birth control pills ). The effect is based on the suppression of ovulation ( ovulation inhibition ).
properties
Nomegestrol acetate is a white to off-white non-hygroscopic crystalline substance and is practically insoluble in water. The octanol / water partition coefficient is 3.70 (at 25 ° C).
Nomegestrol acetate has 6 asymmetric carbon atoms. The configuration on the C8, C9, C10, C13 and on the C14 is given by the ring structure. Nomegestrol acetate shows polymorphism .
Effects
Nomegestrol acetate is derived from progesterone and has a strong affinity for the human progesterone receptor . It has an antigonadotropin, antiestrogenic and slightly antiandrogenic effect.
Nomegestrol acetate has neither estrogenic , androgenic , glucocorticoid nor mineralocorticoid effects.
Absorption and distribution in the body
Orally applied nomegestrol acetate is rapidly absorbed. Maximum plasma nomegestrol acetate concentrations of approximately 7 ng / ml are reached 2 hours after a single dose. The absolute bioavailability of nomegestrol acetate after a single dose is 63%. Food intake showed no clinically relevant effects on the bioavailability of nomegestrol acetate.
Use for disorders of the menstrual cycle
Nomegestrol acetate is used for disorders of the menstrual cycle ( menometrorrhage , secondary amenorrhea , uterine hemorrhage, especially during menopause ), as well as for the treatment of dysmenorrhea and premenstrual syndrome .
Use for contraception
Under the trade name Zoely was the first monophasic combination pill that which occurs in this form in the female body estrogen 17 β - estradiol containing and nomegestrol acetate, in July 2011 in Europe for oral contraception approved. It is taken per cycle over 28 days with 24 active ingredient-containing and 4 active ingredient-free tablets.
Studies
The drug had shown convincing contraceptive safety in women aged 18 to 50 years in a Phase III study (SAMBA) published in the European Journal of Contraception and Reproductive Health Care . The 1,591 users had shorter withdrawal bleeds than the 535 study participants in the comparison group with 3 mg drospirenone (DRSP) / 30 μg ethinylestradiol (EE) and a comparable rate of intermenstrual bleeding and spotting in both study arms .
Adverse effects and restrictions on use
Contraindications for Zoely are existing or previous venous thromboses (deep vein thrombosis , pulmonary embolism ), arterial thrombosis (e.g. myocardial infarction ), prodromes of a thrombosis (e.g. transient ischemic attack , angina pectoris ), existing or previous stroke , migraines with focal a history of neurological symptoms, the presence of one or more serious risk factors for venous or arterial thrombosis such as: diabetes mellitus with vascular changes; severe hypertension ; severe dyslipoproteinemia and unexplained vaginal bleeding. Zoely should not be used during pregnancy .
Interactions between oral contraceptives and drugs that induce enzymes can lead to breakthrough bleeding and even contraceptive failure: e.g. B. phenytoin , phenobarbital , primidone , bosentan , carbamazepine , rifampicin and drugs or herbal preparations containing St. John's wort and, to a lesser extent, oxcarbazepine , topiramate , felbamate and griseofulvin .
Acne, decreased libido , depression / depressed mood, mood swings, headaches, migraines and nausea can occur as undesirable effects (side effects) .
Delivery bottleneck
In summer 2019 there were delivery bottlenecks at Zoely in Germany for several months. The manufacturer justified this u. a. with problems with a packaging line and bottlenecks in the production of active ingredients.
literature
- Stefano Lello: Nomegestrol acetate. Pharmacology, safety profile and therapeutic efficacy. In: Drugs. Volume 70, Issue 5, 2010, pp. 541-559. PMID 20329803 .
Trade names
- Teva Pharmaceutical Industries : Lutenyl (F, B, IT)
- MSD Sharp & Dohme : in a fixed combination with Estradiol : Zoely
Individual evidence
- ↑ a b Datasheet Nomegestrol acetate from Sigma-Aldrich , accessed on March 4, 2012 ( PDF ).
- ↑ Summary of the European public assessment report (EPAR) for Zoely from the European Medicines Agency (EMA).
- ↑ a b c d e f Summary of the product characteristics (PDF; 274 kB) of the EMA (German).
- ↑ External identifiers or database links for nomegestrol acetate : CAS number: 58652-20-3, EC number: 261-379-8, ECHA InfoCard: 100.055.781 , PubChem : 91668 , ChemSpider : 82771 , DrugBank : DB13981 , Wikidata : Q7048519 .
- ↑ Fachinfo Lutenyl (Italian).
- ↑ Contraception: First monophasic pill with estradiol. In: Dtsch Arztebl. 108 (48), 2011, pp. A-2620.
- ↑ D. Mansour et al .: Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17 β -oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. In: The European Journal of Contraception and Reproductive Health Care. 2011. PMID 21995590 .
- ↑ Contraceptives - New Combination. In: Pharmaceutical newspaper. 13/2012, accessed on April 5, 2012.
- ↑ Bottleneck in the pill . In: Der Spiegel . No. 29 , 2019, pp. 66 ( online - July 13, 2019 ).
