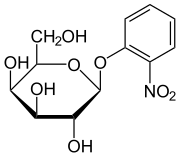
o -nitrophenyl-β- D -galactopyranoside
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | o -nitrophenyl-β- D -galactopyranoside | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 12 H 15 NO 8 | ||||||||||||||||||
| Brief description |
white odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 301.25 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
192 ° C |
||||||||||||||||||
| solubility |
soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
o -nitrophenyl-β- D -galactopyranoside ( ONPG ) is an artificial glycoside and chromogenic substrate for β-galactosidases , e.g. B. the enzyme encodedby the lacZ gene from Escherichia coli .
properties
Certain β-galactosidases hydrolyze ONPG to galactose and the yellow dye o-nitrophenol .
use
In biochemistry and microbiological diagnostics , ONPG is used for the qualitative and quantitative determination of the activity of β-galactosidases, see ONPG test .
The disaccharide lactose is an excellent substrate for β-galactosidase . Similar to X-Gal , which is used for the qualitative determination of β-galactosidase, ONPG is a lactose analog that represents a chromogenic substrate for β-galactosidase.
In one reaction, the hydrolytic cleavage of the colorless ONPG produces galactose and the yellow-colored o-nitrophenol . In order to obtain a pseudo first order reaction, an excess of ONPG must be present in the approach. The intensity of the yellow coloration then only depends on the concentration of β-galactosidase and the duration of the reaction. The reaction is stopped by adding sodium carbonate (shifting the pH value to the basic range (pH 11)), since β-galactosidases, like most enzymes, are inactive at high pH values.
The amount of o -nitrophenol formed is determined by absorption in a photometer at 420 nm . A specific activity of β-galactosidase can be calculated from the reaction time, the absorption value and a value for the biomass used (protein concentration, turbidity of the bacterial culture, etc.).
See also
literature
- JH Miller Assay of β-galactosidase , In: JH Miller Experiments in Molecular Genetics , pp. 352-355, Cold Spring Harbor Laboratory , Cold Spring Harbor, 1972, ISBN 0-87969-106-9 .