Oxyfluorfen
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
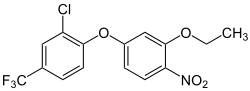
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Oxyfluorfen | ||||||||||||||||||
| other names |
2-chloro-α, α, α-trifluor- p -tolyl-3-ethoxy-4-nitrophenyl ether ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 15 H 11 ClF 3 NO 4 | ||||||||||||||||||
| Brief description |
orange solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 361.70 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.49 g cm −3 |
||||||||||||||||||
| Melting point |
85-90 ° C |
||||||||||||||||||
| boiling point |
358 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Oxyfluorfen is a chemical compound from the diphenylether herbicide group .
Extraction and presentation
Oxyfluorfen can be obtained by reacting 3,4-dichlorobenzotrifluoride with resorcinol and then treating the reaction product with ethanol in the presence of potassium hydroxide .
properties
Oxyfluorfen is an orange solid that is practically insoluble in water. It is stable to hydrolysis at pH values of 5, 7 and 9. Rapid photodegration takes place in solution.
use
Oxyfluorfen is used as a herbicide . In the United States, it has been used for pre- and post-emergence control of annual broadleaf weeds and grasses on a variety of tree crops, nuts, vines, and crops. In agriculture, it is mainly used for grapes and almonds, otherwise also in private ornamental trees and in forestry. Oxyfluorfen is also used for weed control in driveways and similar areas in residential areas. It was first approved in the USA in 1979. It was reviewed by EFSA in 2010. It has been approved in the EU for a limited period until 2021 since 2012.
In Germany, Austria and Switzerland, no pesticides with this active ingredient are permitted.
Individual evidence
- ↑ a b c d e f g h i Entry on Oxyfluorfen in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on November 4, 2016.
- ↑ a b c d e data sheet Oxyfluorfen, PESTANAL at Sigma-Aldrich , accessed on November 4, 2016 ( PDF ).
- ^ Terence Robert Roberts, David Herd Hutson: Metabolic Pathways of Agrochemicals: Herbicides and plant growth regulators . Royal Society of Chemistry, 1998, ISBN 978-0-85404-494-8 , pp. 330 ( limited preview in Google Book search).
- ↑ EPA : Oxyfluorfen RED Facts , accessed November 4, 2016
- ↑ European Food Safety Authority : Conclusion on the peer review of the pesticide risk assessment of the active substance oxyfluorfen . In: EFSA Journal . tape 8 , no. November 11 , 2010, doi : 10.2903 / j.efsa.2010.1906 .
- ↑ europa.eu: L_2011205DE.01000901.xml IMPLEMENTING REGULATION (EU) No. 798/2011 OF THE COMMISSION of August 9, 2011 for the approval of the active ingredient Oxyfluorfen according to Regulation (EC) No. 1107/2009 of the European Parliament and of the Council on the Placing plant protection products on the market and amending the Annex to Commission Implementing Regulation (EU) No. 540/2011 and Commission Decision 2008/934 / EC
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Oxyfluorfen in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on December 7, 2019.
