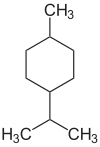
p -Menthan
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | p -Menthan | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 20 | |||||||||||||||
| Brief description |
Liquid smelling like fennel |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 140.27 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
|
|||||||||||||||
| boiling point |
|
|||||||||||||||
| solubility |
almost insoluble in water (0.62 mg l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
p- menthane ( para- menthane ) is the common name of the monocyclic chemical compound 1-methyl-4-isopropyl-cyclohexane from the group of monoterpenes . para- menthane forms the backbone of many naturally occurring terpenes such as terpinene , limonene , phellandrene and terpinolene .
Occurrence
In contrast to other terpenes , menthane occurs only rarely in nature, but has been found in the essential fruit oil of the tree Eucalyptus globulus .
Extraction and presentation
Menthane can be produced by catalytic hydrogenation of the aromatic p -cyymol or the unsaturated compounds limonene and α-terpinene .
properties
Menthane is a colorless liquid with a fennel or mint-like odor. It belongs to the terpene - hydrocarbons . In addition to the para-menthane also are ortho and meta (isomers o - and m -Menthan) known. Only the p However -Menthan there are two stereoisomers , the cis - p -Menthan and trans - p -Menthan. In the cis form, the two substituents point to the front, in the trans form one points towards the front and one towards the back.
use
Menthane or the more effective derivative para- menthane-3,8-diol (PMD) is used as a repellent against ticks and insects such as mosquitoes and industrially as an intermediate in the synthesis of p- menthane hydroperoxide , a catalyst for radical polymerization .
Individual evidence
- ↑ a b c Entry on p-menthane. In: Römpp Online . Georg Thieme Verlag, accessed on July 7, 2014.
- ^ A b c d R. T. O'Connor, LA Goldblatt: Correlation of Ultraviolet and Infrared Spectra of Terpene Hydrocarbons , in: Anal. Chem. 1954, 26, pp. 1726-1737; doi: 10.1021 / ac60095a014 .
- ↑ a b c Entry on 1-isopropyl-4-methylcyclohexane in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Repellants: Protected against ticks, mosquitoes and the like . In: Pharmaceutical newspaper . 2013, No. 24.



