Perfluorooctanesulfonamide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
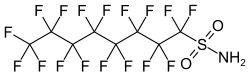
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Perfluorooctanesulfonamide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 2 F 17 NO 2 S | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 499.14 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
145-150 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Perfluorooctanesulfonamide ( PFOSA ) is a chemical compound that belongs to the perfluorinated and polyfluorinated alkyl compounds (PFAS).
In the environment, PFOSA can be converted to perfluorooctane sulfonate (PFOS).
presentation
PFOSA can be prepared by the reaction of perfluorooctanesulfonic acid halides with liquid ammonia or by a two-step reaction via azide , followed by a reduction by Zn / HCl .
use
N- alkyl-substituted perfluorooctanesulfonamides are used in photographic papers, in medical applications and in pesticides . PFOSA accumulates through the food chain ( bioaccumulation ). It is believed that PFOSA is the biologically active form of the insecticide sulfluramide ( N -ethyl perfluorooctanesulfomamide).
regulation
Since perfluorooctanesulfonamide falls under the definition “ Perfluorooctanesulfonic acid and its derivatives (PFOS) C 8 F 17 SO 2 X (X = OH, metal salts (O – M + ), halides , amides and other derivatives including polymers )”, it is subject to the EU and a far-reaching ban in Switzerland.
Individual evidence
- ↑ a b c d JK-Scientific: Perfluorooctanesulfonamide (PDF; 132 kB), accessed on December 27, 2019.
- ^ A b HJ Lehmler: Synthesis of environmentally relevant fluorinated surfactants — a review . In: Chemosphere . 58, No. 11, 2005, pp. 1471-1496. doi : 10.1016 / j.chemosphere.2004.11.078 . PMID 15694468 .
- ↑ Lehmler HJ, Rama Rao VV, Nauduri D, Vargo JD, Parkin S: Synthesis and Structure of Environmentally Relevant Perfluorinated Sulfonamides . In: J Fluor Chem . 128, No. 6, 2007, pp. 595-607. doi : 10.1016 / j.jfluchem.2007.01.013 . PMID 18516235 . PMC 2394736 (free full text).
- ↑ Patent application EP0021003B1 : Use of perfluoroalkanesulfonamide salts as surfactants. Registered on May 14, 1980 , published on February 9, 1983 , applicant: Bayer AG , inventor: Karl-Heinz Mitschke, Hans Niederprüm.
- ↑ UBA Text 41/2007: Development and validation of a method for the determination of polyfluorinated organic substances in sea water, sediments and biota; Investigations into the occurrence of these pollutants in the North and Baltic Seas (PDF; 6.4 MB).
- ↑ AA Starkov KB Wallace: Structural determinants of fluorochemical-induced mitochondrial dysfunction . In: Toxicol. Sci. . 66, No. 2, 2002, pp. 244-252. doi : 10.1093 / toxsci / 66.2.244 . PMID 11896291 .
- ↑ Regulation (EU) 2019/1021 of the European Parliament and of the Council of June 20, 2019 on persistent organic pollutants (new version)
- ↑ Chemical Risk Reduction Ordinance , Appendix 1.16
