Phenapa clay
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phenapa clay | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 11 H 15 Cl 2 O 2 PS 3 | |||||||||||||||
| Brief description |
liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 377.32 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.363 g cm −3 |
|||||||||||||||
| Melting point |
16.2 ° C |
|||||||||||||||
| boiling point |
120 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Phenkapton is a chemical compound from the group of thiophosphoric acid esters .
Extraction and presentation
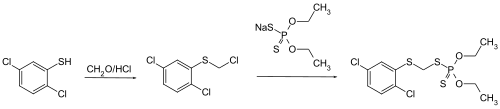
Phenkapton can be produced by reacting 2,5-dichlorothiophenol with formaldehyde and hydrochloric acid by chloromethylation followed by esterification of the intermediate with sodium diethyl phosphorodithionate.
use
Like its derivative methyl phenapa clay (CAS number: 3735-23-7), phenapone clay is used as an acaricide and insecticide .
The World Health Organization classifies phenkapton as an outdated pesticide active ingredient.
In Germany, Austria and Switzerland, no pesticides with this active ingredient are permitted. Phenkapton was approved in the FRG between 1971 and 1974 and in the GDR until 1967.
Individual evidence
- ↑ a b c d e f g h Entry for CAS no. 2275-14-1 in the GESTIS substance database of the IFA , accessed on March 25, 2013(JavaScript required) .
- ↑ Entry on Phenkapton in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ A b G. Matolcsy, M. Nádasy, V. Andriska: Pesticide Chemistry . Elsevier, 1989, ISBN 0-08-087491-6 , pp. 250 ( limited preview in Google Book Search).
- ^ SD Gangolli: The Dictionary of Substances and Their Effects (Dose): Os . Royal Society of Chemistry, 1999, ISBN 0-85404-833-2 , pp. 209 ( limited preview in Google Book search).
- ↑ WHO: Active ingredients believed to be obsolete or discontinued for use as pesticides , in The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2009 (PDF; 2.2 MB).
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 23, 2016.
- ↑ Peter Brandt: Reports on Plant Protection Products 2009: Active Ingredients In Plant Protection Products ... Springer DE, 2010, ISBN 3-0348-0029-0 , p. 23 ( limited preview in Google Book search).