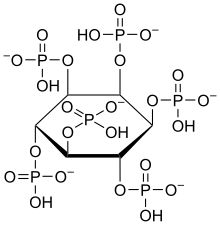
Phytic acid
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | Phytic acid | |||||||||
| other names | ||||||||||
| Molecular formula | C 6 H 18 O 24 P 6 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| ATC code | ||||||||||
| properties | ||||||||||
| Molar mass | 660.04 g mol −1 | |||||||||
| Physical state |
solid (potassium salt) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Phytic acid (hexaphosphoric acid ester of myo - inositol , IP6 ) is one of the bioactive substances. In plants such as legumes , cereals and oilseeds, it serves as a store for phosphate and cations (for potassium, magnesium, calcium, manganese, barium and iron (II) ions), which the seedling needs for growth. Due to its complex-forming properties, it can bind minerals such as calcium , magnesium , iron and zinc in the stomach and intestines insoluble in humans , so that these are no longer available to the body.
Phytic acid occurs naturally as an anion called phytate . Phytate is particularly high in maize, soy and wheat, barley and rye bran ; it is obtained from corn steep water and rice husks . Also in the peanut like other legumes and - - is much phytate included, this is why, despite their high mineral content as a mineral source is only of limited use.
Phytic acid and mineral absorption
Minerals are usually absorbed in the small intestine . Certain food substances can form solid complexes with minerals and thus hinder their absorption into the body. Phytic acid has this property. In addition, calcium , magnesium and zinc are released into the small intestine via the digestive secretions of the pancreas in a recycling process and reabsorbed from there. A high proportion of phytin in the diet can therefore lead to a deficiency in these ions - for example in a diet with a lot of legumes , especially soy , but also whole grains.
Advantages and disadvantages
So far, phytic acid has only been viewed as an undesirable ingredient in food , as the minerals it binds in the plant cannot be absorbed by the body. In the production of whole grains therefore by special dough process of phytin content reduced. However, some representatives of whole food nutrition believe that the property of binding minerals is not a major disadvantage in a balanced mixed diet.
Phytic acid retards z. B. the breakdown of starch in the body. According to some supporters of wholefood nutrition, this could regulate the blood sugar concentration. In addition, phytic acid would bind too much metal ions, especially iron, in food, which would pose an increased risk of colon cancer.
Phytate and manure
Ruminants are the only mammals that can break down phytic acid and utilize the phosphate produced in the process . The bacteria in your stomach produce the enzyme phytase , which breaks down phytate into sugar and phosphate.
The manure of ruminants is therefore low in phosphate, in contrast to the excretion products of other livestock, which can pose a serious disposal and environmental problem with high population densities.
use
Phytic acid and its metal salts are mainly used as complexing agents in the food industry and as a fertilizer additive, for example for hydroponic fertilizers .
Nuclear medicine
The radioactive 99m Technetium phytate in nuclear medicine as a tracer in the liver static scintigraphy used.
Food
The salts calcium phytate and calcium-magnesium phytate are used as clarifying agents for beverages.
restoration
When restoring historical documents, phytate is used to complex the iron in iron gall inks and to stop the oxidation of the cellulose and thus the disintegration of the documents (so-called " ink corrosion ").
Individual evidence
- ↑ a b c data sheet Phytic acid dipotassium salt from Sigma-Aldrich , accessed on June 14, 2011 ( PDF ).
- ↑ E. Strobel, E. Ahrens, G. Hartmann, H. Kluge, H. Jeroch: Content of the ingredients of wheat, rye and oats when grown under conventional and organic farming conditions . In: The soil culture . tape 52 , no. 4 , 2001, p. 221–231 ( full text [PDF; 1.9 MB ]).
- ↑ phytic acid. In: Lexicon of Biochemistry. Spektrum Akademischer Verlag, accessed on June 27, 2018 .
- ↑ Andreas Hahn, Alexander Ströhle, Maike Wolters: Nutrition. Wissenschaftliche Verlagsgesellschaft, Stuttgart 2006, ISBN 3-8047-2293-8 .
- ↑ Hans-Dieter Belitz: Textbook of food chemistry. Springer-Verlag, 2013, ISBN 978-3-662-08308-6 , p. 368 ( limited preview in the Google book search).
- ↑ Additives in the EU (as of December 2011; PDF; 235 kB).
- ↑ Explanation of ink damage and countermeasures in the "Conservation Forum" of the University of Münster with references , last accessed on September 25, 2014.
- ↑ IADA Congress , last accessed on June 10, 2020.
literature
- BF Harland, D. Donald Oberleas: Effects of Dietary Fiber and Phytate on the Homeostasis and Bioavailability of Minerals. In: Gene A. Spiller (Ed.): CRC Handbook of Dietary Fiber in Human Nutrition. Third edition. CRC Press, 2001, ISBN 0-8493-2387-8 , pp. 161-170.
- SB Shears: Assessing the omnipotence of inositol hexakisphosphate. In: Cell. Signal. 13 (3), 2001, pp. 151-158. PMID 11282453
- V. Raboy: myo-inositol-1,2,3,4,5,6-hexakisphosphate. In: Phytochemistry 64 (6), 2003, pp. 1033-1043.
- AR Alcazar-Roman et al: Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. In: Nat Cell Biol . 8 (7), 2006, pp. 711-716. PMID 16783363
- CS Weirich et al .: Activation of the DExD / H-box protein Dbp5 by the nuclear-pore protein Gle1 and its coactivator InsP6 is required for mRNA export. In: Nat. Cell Biol. 8 (7), 2006, pp. 668-676. PMID 16783364