Profenophos
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
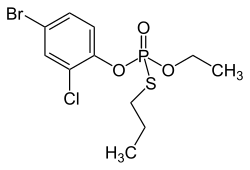
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Profenophos | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 11 H 15 BrClO 3 PS | |||||||||||||||
| Brief description |
yellowish liquid with a garlic-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 373.64 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.455 g cm −3 |
|||||||||||||||
| Melting point |
-76 ° C |
|||||||||||||||
| boiling point |
124 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Profenophos is a chemical compound from the group of thiophosphoric acid esters .
Extraction and presentation
Profenophos can be obtained by reacting o-chlorophenol with phosphorus oxychloride , 1-propanethiol and ethanol .
It can also be made by reacting phosphorus oxychloride with sodium ethoxide , sodium 1-propanethiolate , 4-bromo-2-chlorophenol and sodium hydroxide .
properties
Profenophos is a yellowish liquid with a garlic-like odor. It is stable under neutral and slightly acidic conditions, but hydrolyzes in alkaline media.
use
Profenophos is used as an insecticide mainly for use on cotton . It was first approved in the US in 1982, where approximately 775,000 lbs were used annually in 2005 . Its effect is based on the inhibition of acetylcholinesterase .
Admission
The active ingredient Protofenophos is not approved for use in plant protection products in the European Union. In Germany, Austria and Switzerland, no pesticides with this active ingredient are permitted. Its manufacture and export are still legal, at least in Switzerland (as of 2019).
Web links
- Joint Meeting on Pesticide Residues (JMPR), Monograph for Profenophos
Individual evidence
- ↑ a b c d e f g h Entry on O- (4-bromo-2-chlorophenyl) -O-ethyl-S-propylthiophosphate in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ a b c d e Entry on Profenofos in the Hazardous Substances Data Bank , accessed on June 25, 2012.
- ↑ Entry on O- (4-bromo-2-chlorophenyl) O-ethyl S-propyl phosphorothioate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can use the harmonized classification and labeling expand .
- ↑ Thomas A. Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5 , pp. 332 ( limited preview in Google Book search).
- ^ Terence Robert Roberts, DH Hutson: Metabolic pathways of agrochemicals . Royal Society of Chemistry, 1999, ISBN 978-0-85404-499-3 , pp. 456 ( limited preview in Google Book search).
- ↑ EPA: Reregistration Eligibility Decision for Profenofos (PDF; 874 kB), July 31, 2006.
- ↑ Regulation (EC) No. 2076/2002 of the Commission of November 20, 2002 extending the deadline in accordance with Article 8 (2) of Council Directive 91/414 / EEC and on the non-inclusion of certain active substances in Annex I of this directive as well as the revocation of the Approvals of plant protection products with these active ingredients (PDF)
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Profenofos in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 8, 2016.
- ↑ Stefan Häne: Too toxic for Switzerland - but exportable anyway. In: derbund.ch . March 15, 2019, accessed March 16, 2019 .


