Pyritinol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
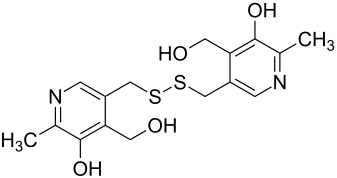
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Pyritinol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 16 H 20 N 2 O 4 S 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 368.47 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
216-217 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Pyritinol (trade name: Encephabol , manufactured by Merck KGaA ) is a drug , known as anti-dementia drug for the treatment of senile dementia is used.
synthesis
The compound can be prepared from pyridoxine in a three-step synthesis . The first step is the conversion of pyridoxine hydrochloride in concentrated hydrobromic acid to the corresponding bromine compound. This is then reacted with potassium ethyl xanthate . The target compound is produced by the dimerization of the xanthate obtained in the presence of ammonia .
Pharmacokinetics
The pyridoxine residues (vitamin B6) resulting from the cleavage at the disulfide bridge are pharmacologically effective . The elimination half-life is approximately 6 hours, with a range of 2.5 to 8 hours. The availability is over 80%. After oral administration (in tablet form), the maximum effect is reached after approx. 30 to 60 minutes. The active ingredient crosses the blood-brain barrier and accumulates in the gray matter. Over 70% of the active substance and the pharmacologically active metabolites and their conjugates are excreted within 24 hours via the kidneys and 5% with the faeces . No accumulation of pyritinol was observed in the organism.
history
The drug Encephabol from Merck with the active ingredient pyritinol was brought onto the market in Germany on May 15, 1963. It was recommended for the treatment of organic brain damage in children. Years before it was approved, it was tested on children, especially children in homes .
Web links
- Pharmaceutical information. Pyritinol. University of Innsbruck, November 27, 1996.
Individual evidence
- ↑ G. Quadbeck, HR Landmann, W. Sachsse, I. Schmidt: The influence of pyrithioxin on the blood-brain barrier . In: Pharmacology . tape 7 , no. 3 , July 1, 2004, p. 144 , doi : 10.1159 / 000135213 .
- ↑ B. Paul, W. Korytnyk: Selective esterifications and acyl rearrangements in vitamin B6 . In: Tetrahedron , 25 (1969) 1071-1087, doi: 10.1016 / S0040-4020 (01) 82680-6 .
- ↑ a b c data sheet pyritinol from Sigma-Aldrich , accessed on October 18, 2016 ( PDF ).
- ^ A b c d e A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications. 4th edition. Thieme 2001, ISBN 3-13-115134-X .
- ↑ G. McCasland, L. Gottwald, A. Furst: 4,5-Dihalo and 3-Amino Analogs of Pyridoxine. New Route to 4-Deoxypyridoxine . In: J. Org. Chem. , 26, 1961, pp. 3541-3543, doi: 10.1021 / jo01067a622 .
- ↑ Patents DE 1,135,460 (E. Merck AG 1958); U.S. 3,010,966 (E. Merck AG 1961).
- ↑ Sylvia Wagner: docserv.uni-duesseldorf.de (PDF) Dissertation
- ↑ spiegel.de
