Quinagolide
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
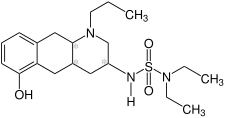
|
|||||||||||||
| Structural formula without information on stereochemistry | |||||||||||||
| General | |||||||||||||
| Non-proprietary name | Quinagolide | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 20 H 33 N 3 O 3 S | ||||||||||||
| Brief description |
|
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class |
Prolactin inhibitors |
||||||||||||
| Mechanism of action |
Agonist at the D 2 receptor |
||||||||||||
| properties | |||||||||||||
| Molar mass |
|
||||||||||||
| Melting point |
|
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Quinagolide is a synthetically produced mixture of two chemical compounds from the groups of decahydroquinoline derivatives and sulfonic acid amides . The drug from the group of dopamine agonists with a non- ergoline structure inhibits the release ( secretion ) of the hormone prolactin and is therefore used to treat abnormally increased prolactin levels in the blood ( hyperprolactinemia ). Quinagolid was developed by Sandoz and a patent applied for in 1983. Usually the more water-soluble hydrochloride is used instead of the free base .
Stereochemistry
Quinagolide is a racemate , i.e. a 1: 1 mixture of the following two enantiomers :
| Enantiomers of quinagolide | |
|---|---|
 CAS number: 140630-79-1 |
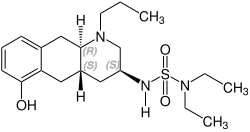 CAS number: 140630-80-4 |
Mechanism and duration of action
Quinagolide binds selectively as an agonist to the D 2 receptors , which are found in the anterior pituitary gland , among others . It strongly inhibits prolactin secretion without lowering the normal levels of the other pituitary hormones. Only the (-) - enantiomer is pharmacologically active .
The effect occurs within 2 hours and reaches its maximum after about 4 to 6 hours. Due to the long plasma half-life of 11 to 17 hours, a single daily intake is sufficient.
Contraindications
The use is contraindicated in case of hypersensitivity to quinagolide and severe impairment of liver and kidney function.
Side effects and interactions
The most common side effects are vomiting, nausea, tiredness, dizziness and headache. Interactions (interactions) have not been studied and are not known. Due to the mechanism of action, interactions with neuroleptics (cancellation of the effect) are conceivable. Alcohol can make quinagolide less tolerable.
preparations
Quinogalide is commercially available in tablet form as Norprolac .
Individual evidence
- ^ A b Franz von Bruchhausen, Hermann Hager, Siegfried Ebel, Eberhard Hackenthal, Ulrike Holzgrabe (eds.): Hager's Handbook of Pharmaceutical Practice: Substances LZ, Volume 5 . Springer, 1999, ISBN 978-3-642-58388-9 , pp. 484–485 ( limited preview in Google Book search).
- ↑ a b c Entry on quinagolid. In: Römpp Online . Georg Thieme Verlag, accessed on July 11, 2013.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Entry on quinagolide in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ Igor Tauveron, Jean-Michel Gesta, Isabelle JALENQUES, Philippe Thiebiot: Acute overdose of a dopamine agonist new, CV 205-502 . In: Clinical Endocrinology . tape 40 , no. 4 , 1994, pp. 551-553 , doi : 10.1111 / j.1365-2265.1994.tb02498.x .
- ↑ EP0077754: Novel pharmaceutically active 1,2,3,4,4a, 5,10,10a-octahydrobenzo (g) quinoline derivatives . Release date April 27, 1983.
- ↑ Rote Liste Service GmbH (Ed.): Rote Liste 2017 - drug directory for Germany (including EU approvals and certain medical devices) . Rote Liste Service GmbH, Frankfurt / Main, 2017, edition 57, ISBN 978-3-946057-10-9 , p. 214.
- ^ R. Nordmann, A. Widmer: Resolution and absolute configuration of the potent dopamine agonist N, N-diethyl-N '- [(3 alpha, 4a alpha, 10a beta) -1,2,3,4,4a, 5 , 10,10a- octahydro-6-hydroxy-1-propyl-3-benzo [g] quinolinyl] sulfamides . In: Journal of medicinal chemistry . tape 28 , no. October 10 , 1985, p. 1540-1542 , doi : 10.1021 / jm00148a030 , PMID 4045929 .
- ↑ a b c d e Specialist information Norprolac 25 μg / 50 μg / 75 μg / 150 μg tablets. Status: March 2013.