Mifepristone
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
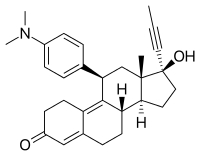
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Mifepristone | |||||||||||||||||||||
| other names |
17 β -hydroxy-11 β - (4-dimethylamino-phenyl) -17 α - (1-propynyl) estra-4,9-dien-3-one |
|||||||||||||||||||||
| Molecular formula | C 29 H 35 NO 2 | |||||||||||||||||||||
| Brief description |
light yellow solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
|
|||||||||||||||||||||
| Mechanism of action |
Antagonist at the progesterone receptor, which leads to degeneration of the uterine mucosa and disruption of placental function |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 429.60 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
192-196 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Mifepristone is a progesterone and glucocorticoid receptor antagonist . It inhibits the action of the progestogen progesterone characterized that it has a five times higher affinity for the receptors of this hormone has, but does not trigger any action at the receptor. It also blocks the receptors for glucocorticoids with a three-fold higher affinity for dexamethasone .
In gynecology , mifepristone is used in combination with the prostaglandins gemeprost or misoprostol to induce an abortion . It is the active ingredient of the first so-called abortion pill RU-486 , which is now marketed under the name Mifegyne . Mifepristone is also used as a long-term therapeutic agent for hypercortisolism in the context of Cushing's syndrome .
application areas
Termination of pregnancy
Mifepristone is the active ingredient in the "abortion pill" (trade name: Mifegyne ). Mifepristone is an inexpensive alternative to gynecological surgery and is associated with fewer health risks for the women affected, especially in poorer countries (e.g. infection) . The abortion pill should not be confused with the morning-after pill .
The intake of mifepristone in the pregnancy takes within 48 hours to open the cervix and to detachment of endometrium . After 36 to 48 hours, the pregnant woman uses a prostaglandin , such as Gemeprost or Misoprostol . The prostaglandin tablets are either swallowed or inserted into the vagina. This causes the uterus to contract, an artificial abortion is triggered, and the fruit is expelled. Mifepristone is effective throughout pregnancy, but studies have shown that it has the highest success rate when taken before the 49th day. The effect on the cervix is also given in non-pregnant women and is used before certain gynecological interventions.
Cushing's Syndrome
In the treatment of Cushing's syndrome, mifepristone is said to act as a glucocorticoid receptor antagonist. The blockade of the receptor suppresses the binding of the natural ligand cortisol and consequently its effect.
It is also used to treat (secondary) hyperglycaemia , when the increased blood sugar level is caused by high levels of cortisol in the blood (hypercortisolism). This occurs in the context of endogenous Cushing's syndrome.
Development and late approval
From 1980 RU-486 was developed by the pharmaceutical laboratory Roussel Uclaf (RU; today part of sanofi-aventis ) against considerable resistance from Hoechst AG, which in 1974 acquired 56% of the second largest French pharmaceutical company. Even before it was approved, it caused a sensation - parts of the women's movement welcomed the development of the active ingredient, while opponents of abortion ran a storm against it. First, France's Minister for Solidarity, Health and Social Protection, Evin, approved the use of mifepristone in around 800 abortion clinics in the country on September 23, 1988. One month after the market launch, the board withdrew it for political reasons (reactions from religious circles) and it was only available again on a limited basis by order of the health minister (36.25% belonged to the state). Even for research into further indications, mifepristone was almost never given worldwide. In Germany, Hoechst AG persistently refused to apply for approval of its active ingredient. a. that they fear a boycott of their products.
See the beginning of the 1990s: Worsening women in the former GDR under reunification through Section 218
In 1997 Hoechst Marion Roussel transferred the rights to the active ingredient free of charge to the former RU board member and inventor Edouard Sakiz . In 1998 it was approved in Great Britain and Sweden. Mifepristone was approved in Germany on November 22, 1999. Switzerland and other European countries followed, as well as the USA in September 2000.
The use for termination of pregnancy was originally approved up to the 7th week of pregnancy (49th day) after the start of the last menstruation , in Great Britain and Sweden until the 63rd day. In 2007, the EU Commission ordered the member states to change their approval, with which the area of application for termination of pregnancy up to the 9th week (63rd day) - with subsequent use of a prostaglandin analogue - was standardized across the EU. In Switzerland, the approval is still valid until the 49th day. For medically necessary abortions, mifepristone is also approved later in pregnancy and is currently (in combination with misoprostol ) the most efficient method. However, there are many so-called off-label uses: up to the 12th week, different dosages, other indications such as endometriosis (growth of the uterine lining) or as a morning-after pill .
According to Section 47a of the German Medicines Act , this medicinal product may only be dispensed directly to certain facilities in which abortions are carried out and only on prescription from a doctor treating there. It must not be marketed through the pharmacy . In Austria , mifepristone has also been allowed to be dispensed by established gynecologists for the purpose of abortion since July 2020.
Contraindications
Treatment with the active ingredient is not allowed in patients with kidney failure or liver failure , chronic kidney weakness, severe or inadequately treated bronchial asthma , or severely underweight . Mifepristone may no longer be used from the 64th day of pregnancy after the absence of a menstrual period.
Side effects
Most of the side effects are not attributable to mifepristone, but to prostaglandin (e.g. misoprostol), which is also administered during an abortion.
Slight side effects can include nausea, vomiting, diarrhea, headache, hot flashes. Often the uterine contractions cause more or less severe pain. Heavy bleeding occurs in about 5% of cases and may require curettage in up to 1.4% of cases . Infections have been reported in less than 5% of women. During induction of an abortion in the second trimester or induction of labor for death of the fetus in utero during the third trimester, uterine rupture has been reported on rare occasions after prostaglandin ingestion. This particularly affected multiple births or patients with a caesarean section scar. Failure rate: 1.3% to 7.5%. A teratogenic effect of Mifegyne (damage to the embryo) cannot be completely ruled out. Misoprostol (Cytotec) accepts it. For this reason, women are strongly advised to have a surgical termination in about 1% of the cases in which the pregnancy continues to develop.
See also
Individual evidence
- ↑ a b c d data sheet Mifepristone from Sigma-Aldrich , accessed on April 10, 2011 ( PDF ).
- ↑ Mutschler, Geisslinger, Kroemer, Schäfer-Korting, Mutschler drug effects, 8th edition, 2001, ISBN 3-8047-1763-2 .
- ↑ Mifepristone (RU486) approved by the FDA in special cases of Cushing's syndrome - German Society for Endocrinology
- ↑ Wannachalee, T., Turcu, AF, & Auchus, RJ (2018). Mifepristone in the treatment of the ectopic adrenocorticotropic hormone syndrome. Clinical endocrinology, 89 (5), 570-576. doi : 10.1111 / cen.13818 PMID 30019523
- ↑ Pivonello, R., De Martino, MC, De Leo, M., Simeoli, C., & Colao, A. (2017). Cushing's disease: the burden of illness. Endocrine, 56 (1), 10-18 doi : 10.1007 / s12020-016-0984-8 PMID 27189147
- ↑ Hexal subsidiary sells Mifegyne pharmische-zeitung.de , accessed on November 24, 2018
- ↑ NOW ALSO IN GERMANY: MIFEPRISTON arznei-telegramm.de, on November 19, 1999, accessed on November 24, 2018
- ↑ Mifegyne (active ingredient mifeprostone): The EU Commission decides to change the product information of the drug , BfArM March 10, 2008.
- ^ "Abortion pill" Mifegyne is becoming more accessible in Austria. Der Standard , July 2, 2020
Web links
- The abortion pill - comparison of surgical abortion and abortion pill on the website of the Swiss Association for the Impunity of Abortion
- profamilia.de - Information from pro familia