Reagent according to Busch
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
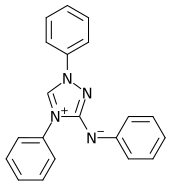
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Reagent according to Busch | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 20 H 16 N 4 | |||||||||||||||
| Brief description |
light and air sensitive yellowish green powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 312.37 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
189-190 ° C |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
" Reagent according to Busch " or more precisely " Reagent for nitric acid according to Busch " are common names for a solution of a chemical compound from the group of triazoles , which was named after its discoverer, the chemist Max Busch . The name nitrone is also common , although the reagent does not belong to the substance group of nitrones and is structurally significantly different from these.
properties
Nitrone (3-anilino-1,4-diphenyl-1,2,4-triazolium, C 20 H 16 N 4 ) is a yellowish powder, forms a zwitterion (“ mesoion ” or “mesomeric betaine”) and is soluble in alcohol, benzene and dilute acetic acid to form the acetate of nitrone. This is used to detect nitrate ions, whereby the nitrate of the nitrone (C 20 H 16 N 4 · HNO 3 ) is formed, which is practically insoluble.
use
To detect nitrates, add nitron acetate solution to the sample solution acidified with dilute sulfuric acid while warming, whereby a whitish precipitate forms. This method was also used for the quantitative detection of nitrates, whereby the precipitate is weighed after filtering off and drying ( gravimetric method ). However, nitrite , bromide , iodide , perchlorate and hexafluorophosphate ions, which together with nitrone also form poorly soluble precipitates, have a disruptive effect . Due to the large number of possible interfering influences - even chloride can only be tolerated in small amounts - the determination by means of nitrone is only accurate in the absence of many common ions. Sample pretreatment in which interfering anions are precipitated, oxidized or reduced may therefore be necessary. In the case of pure, concentrated nitrate solutions, the determination with nitrone is fairly accurate, since over 99.5% of the nitrate is precipitated. The method is not suitable for the micro-scale.
The reaction with hexafluorophosphate to form a precipitate was used for the gravimetric determination of PF 6 - . This was the standard method for determining this ion until 1963.
literature
- Entry on Nitron. In: Römpp Online . Georg Thieme Verlag, accessed on January 2, 2015.
- Gerhard-Otfried Müller: Practical course in quantitative chemical analysis, S. Hirzel Verlag Leipzig, 8th edition 1966, p. 353.
Individual evidence
- ↑ a b Nitron data sheet (PDF) from Merck , accessed on April 22, 2011.
- ↑ a b c data sheet Nitron at Sigma-Aldrich , accessed on May 20, 2017 ( PDF ).
- ↑ Max Busch : Gravimetric determination of nitric acid . In: Reports of the German Chemical Society . tape 38 , no. 1 , January 1, 1905, p. 861-866 , doi : 10.1002 / cber.190503801149 .
- ^ Emanuel Merck: Merck's reagent directory . Contains the common reagents and reactions, sorted by author name. Third edition. Springer-Verlag, Berlin Heidelberg February 1913, p. 55 ( limited preview in Google Book search).
- ↑ a b c d William John Williams: Handbook of Anion Determination . Butterworth-Heinemann, London Boston Sydney a. a. 1979, ISBN 0-408-71306-2 , nitrates, pp. 123–124 ( limited preview in Google Book search).
- ↑ Akiharu Hioki, Tsutomu Watanabe, Kuniko Terajima, Noriko Fudagawa, Masaaki Kubota: Accuracy in Gravimetric Determination of Nitrate and Nitrite as Nitron Nitrate . In: Analytical Sciences . tape 6 , no. 5 , 1990, pp. 757-762 , doi : 10.2116 / analsci.6.757 ( jst.go.jp ).
- ^ William John Williams: Handbook of Anion Determination . Butterworth-Heinemann, London Boston Sydney a. a. 1979, ISBN 0-408-71306-2 , Hexafluorophosphate, p. 428 ( limited preview in Google Book search).
