Rhenium (III) iodide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
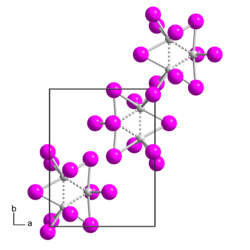
|
||||||||||||||||
| __ Re 3+ __ I - | ||||||||||||||||
| Crystal system |
monoclinic |
|||||||||||||||
| Space group |
P 2 1 / m |
|||||||||||||||
| Lattice parameters |
a = 923.4 pm, b = 1130.9 pm, c = 879.9 pm, β = 110.25 ° |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Rhenium (III) iodide | |||||||||||||||
| other names |
Rhenium triiodide |
|||||||||||||||
| Ratio formula | ReI 3 | |||||||||||||||
| Brief description |
black solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 566.92 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
6.37 g cm −3 |
|||||||||||||||
| Melting point |
800 ° C (decomposition) |
|||||||||||||||
| solubility |
Slightly soluble in water and dilute acids |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Rhenium (III) iodide is an inorganic chemical compound of rhenium from the group of iodides .
Extraction and presentation
Rhenium (III) iodide can be obtained by reacting a perrhenic acid solution with hydriodic acid and ethanol or methanol .
It can also be achieved by thermal decomposition of rhenium (IV) iodide at 350 ° C
or by reaction of rhenium (III) chloride with boron (III) iodide at 310 ° C.
properties
Rhenium (III) iodide is a black, shiny solid with needle-like crystals, which is sparingly soluble in water and dilute acids . It is almost insoluble in methanol , ethanol , ether, petroleum ether and carbon tetrachloride . It is soluble in liquid ammonia with solvolysis . In a vacuum, it slowly decomposes, especially at elevated temperatures, releasing iodine . It breaks down into rhenium and iodine when heated to 800 ° C. Rhenium (III) iodide has a monoclinic crystal structure with the space group P 2 1 / m (space group no. 11) and the lattice parameters a = 923.4 pm, b = 1130.9 pm, c = 879.9 pm and β = 110.25 °. The crystal structure consists of Re 3 I 9 units (island structure), which are linked by Re-I-Re bridges to form zigzag chains.
Individual evidence
- ↑ a b c d e f g h Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1615.
- ↑ a b c data sheet Rhenium (III) iodide, 97% from Sigma-Aldrich , accessed on July 28, 2013 ( PDF ).
- ↑ Hans Peter Latscha, Martin Mutz: Chemistry of the elements . Springer, 2011, ISBN 3-642-16915-5 , pp. 239 ( limited preview in Google Book search).


