Ricinine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
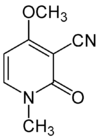
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ricinine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 8 N 2 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 164.16 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
201 ° C |
||||||||||||||||||
| solubility |
soluble in water (2.7 g l −1 ) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ricinine is a weakly toxic ingredient from the Rizinusbaum ( Ricinus communis ), it belongs to the group of pyridine - alkaloids and is adjacent to the highly toxic polypeptide ricin responsible for the toxicity of the plant. Ricinin is found in all parts of the plant and has, among other things, insecticidal effects. In humans, it attacks the liver and kidneys and can cause fatal poisoning. The seeds contain about 0.2% of the alkaloid. In contrast to ricin, ricinin cannot be neutralized by heat treatment. Therefore, in order to use the press cake of the castor kernels as animal feed, ricinin must be removed by laborious extraction after the ricin has been destroyed , see also castor oil .
Ricinine can be produced synthetically by cyclization of 1,1-dicyano-4- ( N , N -dimethylamino) -2-methoxy-1,3-butadiene and subsequent methylation . In the plant it is produced biosynthetically via nicotinamide as an intermediate stage.
Extraction from the plant
Like most alkaloids, ricinine can also be easily isolated from the plant material. After extracting the freshly crushed seedlings from the castor seeds with chloroform and washing the chloroform phase with ammonia solution , a residue with a high content of ricinine is obtained after evaporation. After removing the fats and lipids with diethyl ether , pure ricinine is obtained by repeated dissolution and recrystallization from chloroform.
Analytics
The reliable detection and quantification of ricinin is achieved by GC / MS analysis after adequate sample preparation of the matrices to be examined.
literature
- M. Mittelbach, G. Kastner, H. Junek; Ricinin - easily synthesized. In: Monthly magazine for chemistry. 115 (12), pp. 1467-1470, 1984, doi: 10.1007 / BF00816346 .
- Entry to ricinine. In: Römpp Online . Georg Thieme Verlag, accessed on October 6, 2014.
- GR Waller, LM Henderson: Biosynthesis of the Pyridine Ring of Ricinine. In: J. Bio. Chem. 236 (4), 1961, PMID 13782834 , full text (PDF; English).
Web links
- Ernst Späth, Georg Koller: The synthesis of ricinin. In: Reports of the German Chemical Society (A and B Series). 1923, Volume 56, Issue 11, pp. 2454-2460, doi: 10.1002 / cber.19230561125 .
Individual evidence
- ^ Entry on ricinine. In: Römpp Online . Georg Thieme Verlag, accessed on October 6, 2014.
- ↑ a b Entry on ricinine in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 31, 2018 or earlier.
- ↑ There is not yet a harmonized classification for this substance . A labeling of ricinine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on March 15, 2019, is reproduced from a self-classification by the distributor .
- ^ Karl Paech: Biochemistry and Physiology of Secondary Plant Substances. Springer, 1950, ISBN 978-3-662-27790-4 (reprint), p. 222.
- ↑ Marco Soave: About Ricinin. In: Chemisches Zentralblatt . Vol. 66, Vol. I, 1895, p. 853, Textarchiv - Internet Archive .
- ↑ HU Melchert, E. Pabel: Reliable identification of trichothecenes and other mycotoxins by electron impact and chemical ionization-gas chromatography-mass spectrometry, using an ion-trap system in the multiple mass spectrometry mode. Candidate reference method for complex matrices. In: J. Chromatogr. A . , 12, 1056 (1-2), 2004, pp. 195-199, PMID 15595550 .
