Rosenthal reagent
The Rosenthal reagent (also known as the Rosenthal complex ) is a metallocene -bis (trimethylsilyl) acetylene complex , where the central atom of the metallocene fragment Cp 2 M zirconium ( Cp 2 Zr ) or titanium ( Cp 2 Ti ) can be used . In addition, other ligands (e.g. pyridine ) are often used. With zirconium as the central atom and pyridine as the ligand, a dark purple to black solid is formed which is sensitive to oxygen and moisture and melts at 125–126 ° C with decomposition. If titanium is used, a gold-shimmering solid with a melting point of 81-82 ° C. is obtained. With the help of the reagent, the otherwise unstable metallocene fragments can be generated under mild conditions and made available for further reactions.
The reagent is named after the German chemist Uwe Rosenthal (* 1950), who first synthesized it in 1995 with his colleagues.
Manufacturing
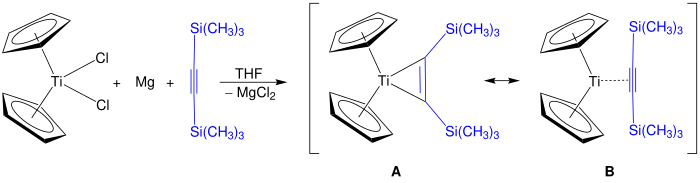
The Rosenthal reagent can be synthesized by reducing titanocene or zirconocene dichloride with magnesium in the presence of bis (trimethylsilyl) acetylene in THF . The reaction product shown for titanocene, the resonance structures A and B on. When using zirconium as the central atom, additional ligands, e.g. B. pyridine, required to stabilize the reagent.
Application and reactions
One of the main areas of application is the synthesis of complex organic structures such as macrocycles and heterometallacycles, which can be obtained selectively and in high yields using the Rosenthal reagent .
Nowadays, the Rosenthal reagent is often used instead of the Negishi reagent (1-butene) zirconocene to generate zirconocene fragments, as it has several decisive advantages. In contrast to the Negishi reagent, the Rosenthal reagent is stable at room temperature and can be stored in an inert environment. This allows a much more precise control over the stoichiometry of the reactions, especially since the unstable Negishi reagent cannot be formed quantitatively. It is thus possible to carry out stoichiometric and catalytic reactions which are influenced by the ligands , metals and substrate substituents used. While titanium complexes react dissociatively , zirconium complexes prefer the associative reaction. A combination of these organometallic complexes with different substrates (e.g. carbonyl compounds , acetylenes , imines , azoles etc.) often leads to new bond types and reactivities. Of particular interest here are novel C — C coupling reactions that enable the formation of heterometallacycles. Pyridine and bis (trimethylsilyl) acetylene are mostly obtained as by-products of coupling reactions with the Rosenthal reagent . These are soluble and volatile , which makes separation from the reaction product much easier.
Individual evidence
- ↑ a b Julian Linshoeft: Rosenthal's Zirconocene . In: Synlett . tape 25 , no. 18 , 2014, p. 2671-2672 , doi : 10.1055 / s-0034-1379317 .
- ↑ a b Uwe Rosenthal, Andreas Ohff, Wolfgang Baumann, Annegret Tillack, Helmar Görls: Structure, properties and NMR spectroscopic characterization of Cp 2 Zr (pyridine) (Me 3 SiC ≡ CSiMe 3 ) . In: Journal of Inorganic and General Chemistry . tape 621 , no. 1 , January 1995, p. 77-83 , doi : 10.1002 / zaac.19956210114 .
- ↑ Uwe Rosenthal, Vladimir V. Burlakov: Organometallic Chemistry of Titanocene and Zirconocene Complexes with Bis (trimethylsilyl) acetylene as the Basis for Applications in Organic Synthesis . In: Titanium and Zirconium in Organic Synthesis . Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, FRG, ISBN 3-527-30428-2 , p. 355-389 , doi : 10.1002 / 3527600671.ch10 .
- ↑ a b A. Ohff, S. Pulst, C. Lefeber, N. Peulecke, P. Arndt: Unusual Reactions of Titanocene- and Zirconocene-Generating Complexes . In: Synlett . tape 1996 , no. 2 , 1996, p. 111-118 , doi : 10.1055 / s-1996-5338 .
- ↑ Uwe Rosenthal, Vladimir V. Burlakov, Perdita Arndt, Wolfgang Baumann, Anke Spannenberg: The Titanocene Complex of Bis (trimethylsilyl) acetylene: Synthesis, Structure, and Chemistry † . In: Organometallics . tape 22 , no. 5 , March 2003, p. 884-900 , doi : 10.1021 / om0208570 .
- ↑ a b c U. Rosenthal: Reactions of group 4 metallocene complexes of bis (trimethylsilyl) acetylene with nitriles and isonitriles . In: Angewandte Chemie . 23 August 2018, doi : 10.1002 / anie.201805157 .
- ↑ a b c Jonathan R. Nitschke, Stefan Zürcher, T. Don Tilley: New Zirconocene-Coupling Route to Large, Functionalized Macrocycles . In: Journal of the American Chemical Society . tape 122 , no. 42 , October 2000, p. 10345-10352 , doi : 10.1021 / ja0020310 .
- ↑ Uwe Rosenthal, Paul-Michael Pellny, Frank G. Kirchbauer, Vladimir V. Burlakov: What Do Titano- and Zirconocenes Do with Diynes and Polyynes? In: Accounts of Chemical Research . tape 33 , no. 2 , February 2000, p. 119-129 , doi : 10.1021 / ar9900109 .