Tetraacetyl ethylenediamine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetraacetyl ethylenediamine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 16 N 2 O 4 | |||||||||||||||
| Brief description |
beige crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 228.25 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
149-154 ° C |
|||||||||||||||
| solubility |
poorly soluble in water (1 g / l at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tetraacetylethylenediamine (TAED) is a tertiary amide that is used as an activator for bleach in laundry detergents . Since TAED is not long-term stable in an aqueous medium, it is used exclusively as granulate in solid and as a suspension in anhydrous liquid detergent preparations.
Occurrence
Tetraacetylethylenediamine is a commonly used ingredient in various detergents. TAED belongs to a larger group of active, uncharged N- and O-acyl compounds (such as tetraacetylglycoluril or glucose penta-acetate) and has a dominant market position with a total production volume of over 80,000 tons / year in Europe compared to anionic compounds that have been developed in the meantime (e.g. Sodium nonanoyloxybenzenesulfonate (NOBS) and cationic (e.g. nitrile quats) bleach activators claimed. Powdered universal detergents contain 1–3% TAED, while so-called detergent concentrates contain 4–6% and compact detergents 6–8% TAED.
TAED achieved a high level of awareness among the population in the late 1970s through a commercial for the detergent OMO, the slogan of which was: " OMO, with TAED system ".
Manufacturing
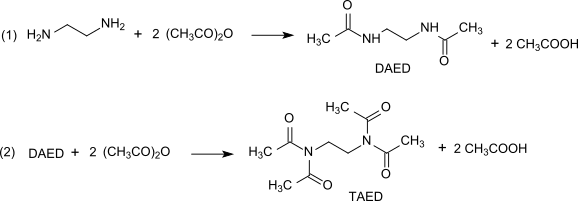
TAED is produced in a two-step process from ethylenediamine and acetic anhydride .
Reaction (1) yields the symmetrically diacetylated intermediate DAED. Reaction (2) at temperatures from 120 to 170 ° C with an excess of acetic anhydride leads to an equilibrium distribution between TAED, diacetylethylenediamine (DAED) and triacetylethylenediamine and produces undesirable dark brown impurities. To obtain a pure, white and odorless product, the reaction mixture is decolorized with activated charcoal, the TAED is isolated by vacuum distillation or the TAED, which is obtained in crystalline form after the mixture has cooled, is washed with acetic acid and water and dried. The reaction carried out on an industrial scale is practically quantitative. The acetic acid obtained in considerable quantities in the reaction can be purified by liquid-liquid extraction or distillation and returned to the production of acetic anhydride.
properties
Because of the unsatisfactory long-term stability of the powdered TAED in solid detergent preparations, it is granulated with the aid of the sodium salt of carboxymethyl cellulose (Na-CMC) and the granulate is sometimes coated or colored blue or green. In spite of the TAED's relatively low solubility in cold water (1 g / l at 20 ° C), the granulate dissolves very quickly and completely in the washing liquor due to its low concentration in the washing powder . In the so-called perhydrolysis, the reaction with hydrogen peroxide from the bleaching agent percarbonate under the conditions of a washing process (pH 10) already at 23 ° C, TAED breaks down to> 99% to form DAED, which is relatively stable to hydrolysis, but easily biodegradable. The peroxyacetic acid formed has bactericidal, virucidal and fungicidal properties and quickly destroys> 99.99% of the microorganisms. The combination of TAED with percarbonate has a disinfecting and deodorizing effect.
use
TAED acts in detergents as an activator of bleaches such as B. sodium perborate or sodium percarbonate . Such peroxidic compounds only have a bleaching effect above 60 ° C. without TAED. However, the use of TAED enables bleaching even at lower temperatures. This is based on the reaction between TAED (1) and the peroxidic bleach or intermediate hydrogen peroxide with the formation of N, N'-diacetylethylenediamine (2) and peroxyacetic acid (3):
This is the actual bleaching agent due to its decay, which occurs even at room temperature, with the formation of so-called singlet oxygen , an excited, highly reactive oxygen species.
Comparatively small amounts of TAED are also used for bleaching paper and textiles and for producing peracetic acid for disinfection purposes. Because of its disinfecting and deodorizing effect, TAED / percarbonate combinations are also used in dishwashing detergents and denture and braces cleaners.
TAED works better at removing hydrophilic stains such as B. tea, coffee and red wine. Compared to hydrophobic stains such. B. grass, juices and spices (curry), it is less effective. The washing process is pH-controlled by suitable additives in the detergent in order to enable rapid and complete peracid formation from the TAED at an initial pH of 10. The resulting acetic acid lowers the pH value, which accelerates the breakdown of most stains. As a relatively mild oxidizing agent, the peracetic acid produced by the action of TAED (in contrast to the bleaching agent sodium hypochlorite, which is still widespread in the USA ) affects textile dyes and fibers only very little. The effect of TAED is unsatisfactory at temperatures below 40 ° C. However, none of the bleach activators previously described as being active below 40 ° C has even come close to the balanced property profile of TAED.
In water-based liquid detergents, the market volume of which is constantly increasing worldwide, TAED is not stable and would also interact with the bleach. Attempts to separate TAED from the water phase and bleaching agent by compartmentalizing have so far been unsuccessful.
ecology
TAED is largely non-toxic and easily biodegradable. TAED and its precursor DAED have a low aquatic ecotoxicity. TAED shows very low toxicity in all exposure routes, is practically non-irritating to skin and eyes and does not give any evidence of skin sensitization. TAED is neither mutagenic nor teratogenic .
Individual evidence
- ↑ a b c Entry on N, N′-Ethylenbis (N-acetylacetamide) in the GESTIS substance database of the IFA , accessed on June 6, 2008 (JavaScript required)
- ↑ Data sheet N, N, N ′, N′-Tetraacetylethylenediamine from Sigma-Aldrich , accessed on May 29, 2011 ( PDF ).
- ↑ a b HERA Human & Environmental Risk Assessment on ingredients of European household cleaning products: Tetraacetylethylenediamine (TAED), CAS number: 10543-57-4 (PDF; 666 kB), Draft, DECEMBER 2002.
- ↑ a b c Clariant Surfactant Division: The Clean and Clever Way of Bleaching - PERACTIVE ( Memento of the original from July 17, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 865 kB), 08.99
- ↑ a b European patent application EPA 004 919, process for the production of N, N, N ', N'-tetraacetyl-ethylenediamine , inventor: G. Müller-Schiedmayer, R. Aigner, applicant: Hoechst AG, published on October 31, 1979
- ↑ European patent application EPA 0 051 739 A1, process for the preparation of N, N, N ', N'-tetraacetylethylenediamine , inventor: W. Köhler et al., Applicant: BASF AG, published on May 19, 1982
- ↑ European patent EP 0 238 958 B1, process for the purification of tetraacetylethylenediamine , inventor: K. Köster, F.-J. Carduck, applicant: Henkel KG aA, published June 12, 1991
- ↑ US patent US 5,100,576, Process for the preparation of a readily soluble bleach activator granulate with a long shelf life , inventor: J. Cramer et al., Applicant: Hoechst AG, issued March 31, 1992.
- ↑ US Patent US 6,080,710, Bleach activator compositions , inventor: JD Withenshaw, MA France, Applicant: Warwick International Group, Ltd., issued June 27, 2000, and US Patent US 5,827,447, Liquid Bleach Agent Composition , inventor: Y Tamura et al., Applicant: Kao Corp., issued October 27, 1998.