Tantalum (IV) sulfide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tantalum (IV) sulfide | |||||||||||||||
| other names |
Tantalum disulfide |
|||||||||||||||
| Ratio formula | TaS 2 | |||||||||||||||
| Brief description |
gray solid or black powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 245.08 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
6.86 g cm −3 |
|||||||||||||||
| Melting point |
> 1300 ° C |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tantalum (IV) sulfide is an inorganic chemical compound of tantalum from the group of sulfides , i.e. the transition metal dichalcogenides. In addition to this, at least four other tantalum sulfides (Ta 6 S, Ta 2 S, Ta 1 + x S 2 and TaS 3 ) are known. Many of them have areas of homogeneity or differ from one another as stacking variants of a basic grid type.
Extraction and presentation
Tantalum (IV) sulfide can be obtained by reacting tantalum with sulfur at 900 ° C.
It can also be obtained by reacting tantalum (V) oxide with hydrogen sulfide or carbon disulfide .
properties
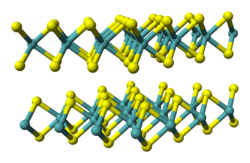
Tantalum (IV) sulfide is a solid that is present at room temperature as a gray metallic or black powder that occurs in several modifications. The form stable at room temperature is referred to as 2H-TaS 2 . It has metallic properties (specific resistance 120 µΩ cm), has a layer structure and is superconducting at 0.75 K. It has a trigonal crystal structure with the space group P 6 3 / mmc (No. 194) isotype to that of niobium (IV ) sulfide . The yellow-looking high-temperature modification 1T-TaS 2 is available from temperatures of 780 ° C. This modification is a semiconductor and can be obtained metastable even at room temperature by quenching. It has an octahedral crystal structure with the space group P 3 m 1 (No. 164) . There is also at least one 3R-TaS 2 , 4H-TaS 2 and one 6R-TaS 2 form.
use
Tantalum (IV) sulfide is used as a lubricant.
Individual evidence
- ↑ a b c d Dale L. Perry: Handbook of Inorganic Compounds, Second Edition . CRC Press, 2011, ISBN 978-1-4398-1462-8 , pp. 410 ( limited preview in Google Book search).
- ^ A b Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer-Verlag, 1998, ISBN 978-3-642-58842-6 , p. 754 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 1470.
- ↑ a b R.MA Lieth: Preparation and Crystal Growth of Materials with Layered Structures . Springer Science & Business Media, 1977, ISBN 978-90-277-0638-6 , pp. 188 ( limited preview in Google Book search).
- ↑ NPCs Board of Consultants & Engineers: Handbook on Rare Earth Metals and Alloys (Properties, Extraction, Preparation and Applications) . ASIA PACIFIC BUSINESS PRESS Inc., 2009, ISBN 81-7833-120-9 , pp. 416 ( limited preview in Google Book search).
- ↑ Anja Schlicht: Normally conducting and superconducting properties of dye intercalation compounds of the host lattice 2H-TaS2 . Herbert Utz Verlag, 1999, ISBN 978-3-89675-473-8 , p. 11 ( limited preview in Google Book search).
- ↑ Erwin Riedel: Modern inorganic chemistry . Walter de Gruyter, 2003, ISBN 978-3-11-017838-8 , pp. 498 ( limited preview in Google Book search).
