Temoporfin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
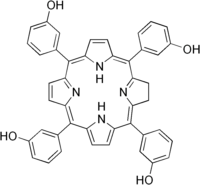
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Temoporfin | |||||||||||||||||||||
| other names |
3,3 ', 3' ', 3' '' - (2,3-dihydroporphyrin-5,10,15,20-tetrayl) tetraphenol ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 44 H 32 O 4 N 4 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
Antineoplastic |
|||||||||||||||||||||
| Mechanism of action |
Formation of reactive oxygen species |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 680.74 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Temoporfin is a photosensitizer ( English photosensitizer ), the chemical to chlorine based and in photodynamic therapy is applied. Temoporfin is used to treat squamous cell carcinoma of the head and neck . It is marketed within the European Union under the name Foscan . The US Food and Drug Administration refused to approve the substance as a medicinal product in 2000. The EU, however, approved Foscan in 2001.
Good treatment results were achieved in 21 of 35 patients in German clinics.
Temoporfin is photoactivated by electromagnetic waves with a length of 652 nm, i.e. in the red range of visible light . Light of this wavelength penetrates the tumor tissue about 10-15 mm deep. Treated patients can remain photosensitive for several weeks after therapy. The effect is based on the formation of reactive oxygen species . The elimination takes place with a half-life of 90 hours instead of the liver.
In order to avoid persistent photosensitivity after administration, efforts are being made to bind the active substance to nanoparticles together with tumor-specific species, such as RGD peptides , and thereby achieve a high level of selectivity.
literature
- S. Marchal, A. Francois, D. Dumas, F. Guillemin, L. Bezdetnaya: Relationship between subcellular localization of Foscan and caspase activation in photosensitized MCF-7 cells . In: British Journal of Cancer . 96, 2007, pp. 944-951, doi: 10.1038 / sj.bjc.6603631 .
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A label of Temoporfin in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on January 12, 2018, is reproduced from a self-classification by the distributor .
- ↑ a b K. Haedicke, D. Kozlova, S. Gräfe, U. Teichgräber, M. Epple, I. Hilger: Multifunctional calcium phosphate nanoparticles for combining near-infrared fluorescence imaging and photodynamic therapy. In: Acta Biomater. 14, 2015, pp. 197-207. doi: 10.1016 / j.actbio.2014.12.009 .
- ^ AE O'Connor, WM Gallagher, AT Byrne: Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. In: Photochemistry and Photobiology. 85, 2009, pp. 1053-1074, doi: 10.1111 / j.1751-1097.2009.00585.x .
- ↑ Foscan approval saves Scotia's skin ( Memento of the original from November 4, 2012 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. , The Scotsman; HighBeam Research.
- ^ Kai Johannes Lorenz, Heinz Maier: Photodynamic therapy with meta-tetrahydroxyphenylchlorin (Foscan®) in the management of squamous cell carcinoma of the head and neck: experience with 35 patients . In: European Archives of Oto-Rhino-Laryngology . tape 266 , no. December 12 , 2009, pp. 1937–1944 , doi : 10.1007 / s00405-009-0947-2 ( PDF ).
- ↑ EPAR: Foscan
- ↑ Hasso Scholz, Ulrich Schwabe (ed.): Pocket book of drug treatment: applied pharmacology . 13th edition. Springer, Berlin / Heidelberg 2005, ISBN 3-540-20821-6 , pp. 843 .