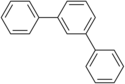
The terphenyls form a group of substances that consist of a central benzene ring and two phenyl radicals connected to one another and thus of three phenyl rings. The different arrangement of the phenyl residues results in three constitutional isomers . As a commercially available product, terphenyl , it is a mixture of the three isomers.
Terphenyls
Surname
o -terphenyl
m -terphenyl
p -terphenyl
other names
1,2-diphenylbenzene
1,3-diphenylbenzene
1,4-diphenylbenzene
Structural formula
CAS number
84-15-1
92-06-8
92-94-4
26140-60-3 (mixture of isomers)
PubChem
6766 7076
7115
Molecular formula
C 18 H 14
Molar mass
230.31 g mol −1
Physical state
firmly
Brief description
white, flammable powder
Melting point
54-56 ° C
84-88 ° C
213 ° C
boiling point
332 ° C
379 ° C
404 ° C
density
1.14 g cm −3
1.23 g cm −3
solubility
Practically insoluble in water
GHS
Caution
Caution
Caution
H and P phrases
302
315 319 335 400
315 319 335 400
no EUH phrases
260 262
273 280 305 + 351 + 338 337 + 313 391
261 273 305 + 351 + 338
MAK
Switzerland: 0.5 ml m −3 or 5 mg m −3
Toxicological data
1900 mg kg −1 ( LD 50 , rat , oral )
2400 mg kg −1 ( LD 50 , rat , oral )
500 mg kg −1 ( LD Lo , rat , oral )
use The most widespread terphenyl is p -terphenyl, which is used as a dye (e.g. as a doping of terrylene ), as a laser material (e.g. for excimer lasers with a fluorescence maximum at 343 nm), as a scintillation counter and in sun creams. o -Terphenyl is used as a synthetic chemical. The polychlorinated terphenyls (PCT) derived from the terphenyls through Suzuki coupling have similar physical-chemical properties as the polychlorinated biphenyls (PCB) and were used, among other things, as heat exchangers and dielectrics in transformers .
Related links Web links Individual evidence
↑ a b c d e f Entry on o-terphenyl GESTIS substance database of the IFA , accessed on April 20, 2017
↑ a b c data sheet m-terphenyl Sigma-Aldrich , accessed on April 20, 2017 ( PDF ).
↑ a b c d e Entry on p-terphenyl GESTIS substance database of the IFA , accessed on April 20, 2017
↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values 26140-60-3 ), accessed on December 8, 2019.
↑ Entry on m-terphenyl
↑ Entry on 1,1 ′: 4 ′, 1 ′ ′ - terphenyl ChemIDplus database of the United States National Library of Medicine (NLM), accessed on December 8, 2019.
↑ A. Pieper, H. Wichmann, M. Bahadir: Synthesis and behavior of polychlorinated terphenyls (PCT) under thermal stress Memento Internet Archive
<img src="https://de.wikipedia.org//de.wikipedia.org/wiki/Special:CentralAutoLogin/start?type=1x1" alt="" title="" width="1" height="1" style="border: none; position: absolute;">