Terpinen-4-ol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
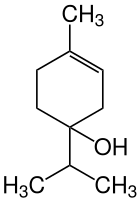
|
||||||||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Terpinen-4-ol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 10 H 18 O | |||||||||||||||||||||
| Brief description |
colorless liquid with a characteristic odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 154.25 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
0.93 g cm −3 |
|||||||||||||||||||||
| boiling point |
211-213 ° C |
|||||||||||||||||||||
| Vapor pressure |
5 h Pa (20 ° C) |
|||||||||||||||||||||
| solubility |
soluble in ethanol |
|||||||||||||||||||||
| Refractive index |
1.4785 (19 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Terpinen-4-ol is a chemical compound that occurs naturally as a secondary plant substance. It is a colorless terpene - alcohol with characteristic floral and spicy smell.
Isomerism
The structure of terpinen-4-ol has a stereocenter, consequently the substance occurs in two stereoisomers or as a mixture thereof. Since only the racemic mixture is used in the perfume industry and the isomers hardly differ, we are only talking about the properties of the mixture here.
Occurrence
Terpinen-4-ol is a component of many essential oils , and occurs, for example, in pine ( pine oil ), eucalyptus ( eucalyptus oil ), lavender ( lavender oil ) and in the tea tree ( tea tree oil ).
Extraction and presentation
Terpinen-4-ol is a by-product in the manufacture of terpineol from terpin hydrate and is thus part of commercial terpineol mixtures.
Pure terpinen-4-ol ( 4 ) can be produced from terpinolene ( 1 ) by photooxidation, reduction of the hydroperoxide formed ( 2 ) and selective hydrogenation of the alcohol formed ( 3 ):
use
Terpinen-4-ol is used in artificial geranium or pepper oils and creates herbal and lavender scents in the perfume industry. It is also used in cosmetic care products ( tea tree oil ).
literature
- H. Surburg and J. Panten: Common Fragrance and Flavor Materials: preparation, properties, and uses . Wiley-VCH, Weinheim 2006, ISBN 3-527-31315-X (English).
Individual evidence
- ↑ Entry on 4-TERPINEOL in the CosIng database of the EU Commission, accessed on May 11, 2020.
- ↑ a b c d data sheet (±) -Terpinen-4-ol (PDF) from Carl Roth , accessed on October 13, 2018.
- ↑ George A. Burdock: Fenaroli's Handbook of Flavor Ingredients . CRC Press, 2004, ISBN 1-4200-3787-0 , pp. 264 ( limited preview in Google Book search).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-358.
- ↑ a b Data sheet (-) - Terpinen-4-ol from Sigma-Aldrich , accessed on October 13, 2018 ( PDF ).
- ↑ R. Hegnauer: Chemotaxonomie der Pflanzen An overview of the distribution and the systematic importance of plant substances . Springer-Verlag, 2013, ISBN 978-3-0348-9389-3 , pp. 359 ( limited preview in Google Book Search).
- ↑ Rudolf Hänsel, Konstantin Keller, Horst Rimpler, Georg Schneider: Hager's handbook of pharmaceutical practice, drugs E-O . Springer-Verlag, 2013, ISBN 978-3-642-57993-6 , pp. 955 ( limited preview in Google Book Search).
- ↑ Ullmann's Encyclopedia of Technical Chemistry . Wiley, 1981, ISBN 3-527-20020-7 , pp. 201 ( limited preview in Google Book search).
- ↑ Peter Brandt: Reports on Food Safety 2008 Federal Monitoring Plan 2008 . Springer-Verlag, 2009, ISBN 978-3-0346-0254-9 , pp. 46 ( limited preview in Google Book search).


