Tetrabromo o -cresol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
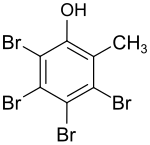
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetrabromo o -cresol | |||||||||||||||
| Molecular formula | C 7 H 4 Br 4 O | |||||||||||||||
| Brief description |
whitish powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 423.72 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
209-212 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tetrabromo- o -cresol is a chemical compound that belongs to the halogen aromatic compounds as well as to the phenols .
Manufacturing
Tetrabromo- o -cresol can be made from o -cresol and elemental bromine in carbon tetrachloride or chloroform in the presence of iron .
properties
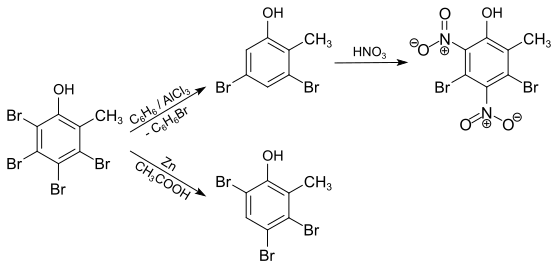
With sodium nitrite in glacial acetic acid , 3,4,5-tribromo-6-nitro- o- cresol is formed , which crystallizes in yellow monoclinic prisms and decomposes at 156 ° C.
The acetyl derivative, which is formed on reaction with acetic anhydride , crystallizes in needles and melts at 154 ° C. The methyl ether, which can be prepared by reacting with dimethyl sulfate , melts at 140.5 ° C. With concentrated nitric acid, tribromo- p -toluchinone is formed in the heat . If the reaction is carried out in the presence of glacial acetic acid and in the cold, an intermediate product is formed which, with ethanolic sodium hydroxide solution in 3,5,6-tribromo-4-nitro- o -cresol , crystallizes in colorless needles which decompose at 177 ° C , can be transferred. With iron (III) chloride , this compound is also oxidized to tribromo- p -toluchinone. Reduction of the nitro compound with tin and hydrochloric acid leads to 3,5,6-tribromo-4- amino - o- cresol .
With benzene in the presence of aluminum chloride, two bromine atoms are split off and 3,5-dibromo- o- cresol is formed. This can easily be nitrated with nitric acid to 3,5-dibromo-4,6-dinitro- o -cresol (melting point 135 ° C). If the debromination is carried out with zinc dust and glacial acetic acid, only one bromine atom is split off; 3,4,6-tribromo- o- cresol is formed.
Individual evidence
- ↑ a b c d data sheet 3,4,5,6-tetrabromo-o-cresol from Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ↑ a b c "Abstracts of Papers on Organic Chemistry", in: Journal of the Chemical Society , 1907 , 42 (1), pp. 125-126; Full text .
- ↑ T. Zincke , A. v. Hedenström: On the effect of bromine and chlorine on phenols. On the action of bromine on o-cresol. , in: Liebigs Ann. , 1905 , 350 , pp. 269-287; doi : 10.1002 / jlac.19063500303 .
- ↑ a b Heilbron: "Dictionary of organic compounds, Volume Four", 1953 , p. 413; Full text .
- ↑ a b "Abstracts of Papers on Organic Chemistry", in: Journal of the Chemical Society , 1907 , 42 (1), pp. 322-323; Full text .
- ↑ a b T. Zincke , W. Klostermann: On the action of nitric acid on halogen derivatives of o-alkylphenols , in: Ber. d. German Chem. Ges. , 1907 , 40 , pp. 679-685; doi : 10.1002 / cber.19070400199 .
- ↑ M. Kohn, M. Weißberg: "About m-Bromphenole. VI. Communication on Bromphenole" in monthly journals for chemistry 1924 , 45 (7-8), pp. 295-303. doi : 10.1007 / BF01521913 .
- ↑ M. Kohn, A. Aron: "Entbromung gebromter Kresole with zinc dust and glacial acetic acid. XXXIV. Communication on Bromphenole" in monthly magazine for chemistry 1932 , 53 (1), p. 48-61. doi : 10.1007 / BF01521772 .