Tetrahydrofurfuryl methacrylate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
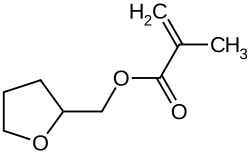
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tetrahydrofurfuryl methacrylate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 14 O 3 | ||||||||||||||||||
| Brief description |
colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 170.206 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.04 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
- 113 ° C |
||||||||||||||||||
| boiling point | |||||||||||||||||||
| Vapor pressure |
0.27 hPa (20 ° C) |
||||||||||||||||||
| solubility |
little in water (19 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tetrahydrofurfuryl methacrylate is a methacrylic acid ester of tetrahydrofuran .
properties
Tetrahydrofurfuryl methacrylate is a chemically unstable liquid with an unpleasant, characteristic odor. It has a flash point of 99 ° C and an ignition temperature of 240 ° C.
Like most acrylic esters, THFMA can also polymerize spontaneously and with a sudden increase in viscosity and temperature ( Trommsdorff effect ) , especially when impurities, oxidizing substances or heavy metals are present, and is therefore brought onto the market in a stabilized manner.
use
Tetrahydrofurfuryl methacrylate is a methacrylate monomer that is used in adhesives . It is contained in the Loctite 330 adhesive, for example . Due to the relatively low glass point of 40 ° C of the (pure) hardened PTHFMA (polytetrahydrofurfuryl methacrylate), the bonded areas have a certain elasticity / toughness and are significantly less brittle than those made of pure PMMA.
Due to the good solvent properties of THFMA, it is also used for the pretreatment of polymer surfaces before bonding, in adhesive formulations and as a reactive thinner in radiation-curing formulations.
PTHFMA absorbs> 70% water and is compatible with copolymers of styrene and allyl alcohol .
Individual evidence
- ↑ a b c d e Entry on tetrahydrofurfuryl methacrylate in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ Datasheet Tetrahydrofurfuryl methacrylate from Sigma-Aldrich , accessed on January 18, 2020 ( PDF ).
- ↑ a b c d e f g Evonik Performance Materials GmbH: Safety data sheet VISIOMER THFMA . No. 9.0 . Darmstadt February 12, 2015.
- ↑ MAK documentation tetrahydrofurfuryl methacrylate . In: Substances harmful to health . 32nd delivery, 2001, doi : 10.1002 / 3527600418.mb245524d0032 .
- ↑ Safety data sheet
- ↑ PD Riggs, M. Braden, DA Tilbrook, H. Swai, RL Clarke, MP Patel: The water uptake of poly (tetrahydrofurfuryl methacrylate) . In: Biomaterials . tape 20 , no. 5 , March 1999, p. 435 , doi : 10.1016 / s0142-9612 (98) 00188-4 (English, PDF ).
- ↑ Patent DE3834599 : Objects made up of compatible polymers. Registered on September 22, 1988 , published on December 7, 1989 , applicant: Röhm GmbH, inventor: Werner Siol.

