Thallium (I) hydroxide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ Tl + __ OH - | ||||||||||||||||
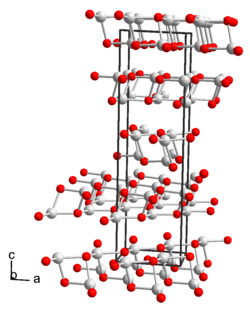
| Crystal system |
monoclinic |
|||||||||||||||
| Space group |
C 2 (No. 5) |
|||||||||||||||
| Lattice parameters |
a = 594.9 pm, b = 622.0 pm, c = 2123.2 pm, β = 91.590 ° |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Thallium (I) hydroxide | |||||||||||||||
| other names |
Thallium hydroxide |
|||||||||||||||
| Ratio formula | Tl (OH) | |||||||||||||||
| Brief description |
yellow solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 221.39 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
7.44 g cm −3 |
|||||||||||||||
| Melting point |
139 ° C (decomposition) |
|||||||||||||||
| solubility |
soluble in water and ethanol |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−239 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Thallium (I) hydroxide is an inorganic chemical compound of thallium from the group of hydroxides .
Extraction and presentation
Thallium (I) hydroxide separates out when thallium ethyl alcoholate is decomposed by water.
This can also be done by direct reaction of thallium with ethanol and oxygen .
Thallium (I) hydroxide can also be obtained by reacting thallium (I) sulfate with barium hydroxide .
properties
Thallium (I) hydroxide is a colorless to yellow solid that is in the form of needles that turn slightly dark in color and are soluble in water and ethanol. Saturated aqueous solutions of the compound attack glass especially in the heat. Thallium (I) hydroxide and its strongly basic aqueous solutions eagerly absorb carbon dioxide with the formation of thallium (I) carbonate . At temperatures of around 140 ° C the compound decomposes with the formation of thallium (I) oxide . The compound reacts with oxygen to form thallium (III) oxide . Thallium (I) hydroxide has a monoclinic crystal structure with the space group C 2 (space group no. 5) and the lattice parameters a = 594.9 pm, b = 622.0 pm, c = 2123.2 pm, β = 91.590 ° .
use
Thallium (I) hydroxide can be used in organic chemistry for Suzuki couplings . It can also be used to detect ozone , since paper soaked with an aqueous solution of the compound turns brown in the presence of traces of ozone.
Individual evidence
- ↑ a b c d e f g h Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 883.
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 93rd Edition . CRC Press, 2012, ISBN 1-4398-8049-2 , pp. 4–94 ( limited preview in Google Book Search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. Walter de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 1398 (reading sample: Part A - Basics of the chemistry of hydrogen. Google book search ).
- ^ A b c Mary Eagleson: Concise Encyclopedia Chemistry . Walter de Gruyter, 1994, ISBN 3-11-085403-1 , p. 1088 ( limited preview in Google Book Search).
- ^ A b Ronald Rich: Inorganic Reactions in Water . Springer, 2007, ISBN 3-540-73962-9 , pp. 321 ( limited preview in Google Book search).
- ↑ Oleg I. Siidra, Sergey N. Britvin, Sergey V. Krivovichev, Wulf Depmeier: Polytypism of Layered alkaline hydroxide: Crystal Structure of TlOH. In: Journal of Inorganic and General Chemistry. 636, 2010, pp. 595-599, doi : 10.1002 / zaac.200900367 .
- ^ Hisashi Yamamoto, Koichiro Oshima: Main Group Metals in Organic Synthesis . John Wiley & Sons, 2006, ISBN 978-3-527-60535-4 , pp. 404 ( limited preview in Google Book Search).


