Density gradient electrophoresis
The Dichtegradientenelektrophorese is an electrophoresis method, which no solid carrier substances used. Therefore it is also called carrier-free electrophoresis. Density gradient electrophoresis was first described by Arne Wilhelm Kaurin Tiselius in 1937.
The passage of the electric current inevitably generated resistance - heat , and this would in a liquid thermal convection effect. In the Dichtegradientenelektrophorese the thermal convection is used electrolyte - solution by a density - gradient prevented.
Density gradient electrophoresis is a particularly gentle method of separating proteins with a high molecular mass from one another, because the proteins do not have to interact with any solid and are therefore not stressed. In contrast to gel electrophoresis, large molecules do not migrate more slowly here because there is no molecular sieve effect. Another advantage of density gradient electrophoresis compared to gel electrophoresis is that the proteins are already in dissolved form after the end of the separation process.
In addition to the proteins, nucleic acids , ribosomes and their subunits, organelles and whole cells can also be separated. For larger particles, such as ribosomes or other organelles, gel electrophoresis methods are not suitable because the mesh size of the gels is too small. Density gradient electrophoresis is a good addition to density gradient centrifugation , because with these two methods different force effects lead to separation in similar buffer media.
The disadvantages of density gradient electrophoresis compared to other separation methods are the higher expenditure of time and lower selectivity.
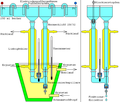
The system shown here is a further developed variant of density gradient electrophoresis from 1972. In contrast to the system by Arne Wilhelm Kaurin Tiselius, the protein mixture to be separated is not at the deepest point of the U-tube, but near the upper end of a density gradient, which allows a higher selectivity at the expense of a smaller amount of protein that can be used.
Sucrose , glycerine , Ficoll or heavy water , for example , can be used as the density-increasing substances for the density gradient . It is necessary that none of the substances which increase the density cause electrical conductivity in aqueous solution. Sucrose and glycerine also have the advantage that they have a stabilizing effect on proteins. Ficoll is well suited for living cells due to its low osmotic activity . Sucrose and glycerine are used for the density gradient in concentrations of 2 to 70 percent (m / V, g / 100ml), at higher concentrations the conductivity would be too much reduced. A density cushion is placed in the lower bend of the U-tube with the help of the highest concentration of the density-increasing substance. The production of a density gradient with the help of a gradient mixer is described under substance gradient .
The electrolyte concentrations should be between 0.02 and 0.05 M , otherwise the conductivity would be too high, which would increase the heat of the current. If too much heat is generated, the density gradient would break up into thermal convection eddies. The thermometer is installed at the lowest point, which is also the place of the highest electrical resistance. At this point, the temperature difference between the density cushion there and the cooling water in the outer jacket should not be more than 2 degrees Celsius . In contrast to the density-increasing substances, the electrolyte concentration is constant in the entire system.
As electrolytes are buffer solutions of salts of weak bases with weak acids particularly favorable because of their decomposition to the electrodes no extreme p H values occur. If the proteins require the addition of halides of the alkali metals , then the strong bases and acids that develop at the electrodes can be kept away from the U-tube and the proteins by separating the electrode buffer as shown above. The electrodes themselves can be made of platinum or graphite .
By choosing the p H -value of the buffer solution, and by the location of positive and negative electrodes, to the running direction and the running can speed influence of the proteins as desired. It is also possible to introduce the protein mixture to be separated with the aid of the density-increasing substance at any point on the density gradient.
It is also possible to use this density gradient system for electrofocusing , which almost always leads to a very high selectivity. The organic ampholyte is only found in the right density gradient, while the density pad below, the left density gradient, and the left electrode cup contain dilute phosphoric acid. In the right electrode cup there is diluted sodium hydroxide solution. This arrangement only applies if the negative electrode is mounted on the top right. Separation of the electrode buffer is not necessary here. A disadvantage of carrier-free electrofocusing is that some proteins become insoluble at the isoelectric point and therefore fail .
After the end of the electrical run, the left branch of the U-tube is closed at the top with a stopper, and the right density gradient is slowly drained through a capillary and fractionated into smaller volumes . When draining after the end of an electrofocusing, it must be ensured that the density cushion containing phosphoric acid remains sufficiently far below the upper end of the capillary, otherwise the proteins will be damaged.