Trifluoromethylsulfur pentafluoride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
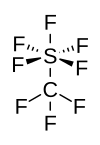
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Trifluoromethylsulfur pentafluoride | ||||||||||||||||||
| Molecular formula | CF 8 S | ||||||||||||||||||
| Brief description |
colorless liquid at −20.4 ° C |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 196.06 g mol −1 | ||||||||||||||||||
| Physical state |
gaseous |
||||||||||||||||||
| Melting point |
−86.9 ° C |
||||||||||||||||||
| boiling point |
−20.4 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
| Global warming potential |
17700 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Trifluoromethylsulfur pentafluoride is a chemical compound from the group of fluorides , which has a very high global warming potential . It was detected in the earth's atmosphere in 2000 . Measurements in Antarctic firn snow layers show that its concentration increased from near zero in the late 1960s to about 0.12 parts per trillion in 1999. The lifetime in the atmosphere is estimated at 1000 years.
Extraction and presentation
Trifluoromethylsulfur pentafluoride can be obtained by reacting methyl mercaptan with cobalt (III) fluoride at 250 ° C or by reacting methyl mercaptan with an excess of fluorine diluted with nitrogen in the presence of copper shavings coated with silver fluoride at 200 ° C. A preparative method that gave the highest yield of trifluoromethylsulfur pentafluoride is the reaction of carbon disulfide with cobalt (III) fluoride at about 250 ° C. It can also be obtained by fluorinating methanol or by reacting bis (trifluoromethyl) disulfide with cobalt (III) fluoride. The preparation by reaction of carbon disulfide with cobalt (III) fluoride or fluorine is also possible, several other derivatives being formed in addition to trifluoromethylsulfur pentafluoride. Scientists suspect that the connection is also created during discharges and switching operations in high-voltage systems that use sulfur hexafluoride to suppress sparks in electrical switchgear.
properties
Trifluoromethylsulfur pentafluoride is a gas that only reacts quickly with alkali metals in red heat . No evidence of the compound's reaction with sodium hydroxide at room temperature was found. Trifluoromethylsulfur pentafluoride is a very good insulator at low pressure, but it decomposes to carbon tetrafluoride and sulfur tetrafluoride by arcing . Trifluoromethylsulfur pentafluoride boils at −20.4 ° C, melts at −86.9 ± 0.2 ° C and has a transition point at −153.3 ± 0.3 ° C. It has a very high absorption capacity for thermal radiation.
literature
- Kiryl Batvinyeu: Development of a new method for measuring SF5CF3 , Institute for Environmental Physics, Ruprecht-Karls-Universität Heidelberg, 2012
Individual evidence
- ↑ a b c d e f Gene A. Silvey, George H. Cady: Trifluoromethylsulfur Pentafluoride. In: Journal of the American Chemical Society. 72, 1950, p. 3624, doi : 10.1021 / ja01164a084 .
- ↑ a b SynQuest Labs, Inc .: Trifluoromethylsulfur pentafluoride | SynQuest Labs, Inc. , accessed November 1, 2018
- ↑ Code of Federal Regulations: Code of Federal Regulations , accessed November 1, 2018
- ↑ Eizi Hirota, Yoshiyuki Kawasima, Ken Ajiki: Internal rotation in trifluoromethylsulfur pentafluoride: CF 3 SF 5 by Fourier transform microwave spectroscopy. In: Journal of Molecular Spectroscopy. 342, 2017, p. 100, doi : 10.1016 / j.jms.2017.06.015 .
- ↑ WT Sturges: A Potent Greenhouse Gas Identified in the Atmosphere: SF5CF3. In: Science. 289, p. 611, doi : 10.1126 / science.289.5479.611 .
- ^ Martin Suen: Trifluoromethyl Sulfur Pentafluoride (CF3SF5): A Review of the Recently Discovered Super-Greenhouse Gas in the Atmosphere. In: The Open Atmospheric Science Journal. 2, 2008, p. 56, doi : 10.2174 / 1874282300802010056 .
- ↑ US EPA: Document Display | NEPIS | US EPA , accessed November 1, 2018.
- ↑ Norman Kharasch, Cal Y. Meyers: The Chemistry of Organic Sulfur Compounds . Elsevier, 2013, ISBN 978-1-4831-5611-8 , pp. 178 ( limited preview in Google Book search).
- ↑ a b innovations-report.de: New greenhouse gas of industrial origin found in the atmosphere , accessed on November 1, 2018

