Trifluoronitrosomethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
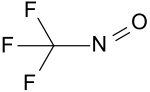
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Trifluoronitrosomethane | |||||||||||||||
| Molecular formula | CF 3 NO | |||||||||||||||
| Brief description |
intense blue gas |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 99.012 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| boiling point |
−86.6 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Trifluoronitrosomethane ( TFNM ) is a highly reactive, toxic gas. It is an intense blue at room temperature.
history
TFNM was first manufactured in 1936 by Otto Ruff and Manfred Giese at the University of Breslau . It was obtained from the fluorination of silver cyanide in the presence of silver nitrate and silver (I) oxide .
properties
Trifluoronitrosomethane has an intense blue color, which is extremely unusual for gases. TFNM dimerizes to gaseous pale yellow 1,2-bis (trifluoromethyl) -diazene-1,2-dioxide through UV radiation.
The bond angle at the nitrogen atom of the TFNM is 112.4 °. The absorption maxima are at 665 and 683 nm . With hydrogen peroxide or molecular oxygen at 100 ° C TFNM can be oxidized to the likewise gaseous colorless trifluoronitromethane . With activated charcoal or in alkaline, trifluoronitrosomethane disproportionates to hexafluoroazoxymethane and trifluoronitromethane.
On double bonds , for example perhalogenated alkenes , to TFNM added to form high molecular weight polymers .
Manufacturing
Trifluoronitrosomethane can be produced from trifluoroiodomethane (CF 3 I) and nitrogen monoxide (NO) using UV light and catalytic amounts of mercury . At normal pressure the yield is up to 90%. In the plasma , CF 3 NO can also be produced from hexafluoroethane or methyl bromide and nitrogen monoxide. TFNM can also be obtained from nitrosyl chloride and trifluoroacetic anhydride or silver trifluoroacetate . At first CF 3 COONO is formed, which decomposes into carbon dioxide and TFNM when heated.
Individual evidence
- ↑ a b c J. Jander and RN Haszeldine: About the CF 3 NO and some of its reactions. In: Die Naturwissenschaften 40, 1953, pp. 579-579. doi : 10.1007 / BF00594731 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ O. Ruff and M. Giese: Das Trifluor-nitroso-methan, CF 3 NO (III.) In: Ber dtsch Chem Ges 69, 1936, pp. 684-689. doi : 10.1002 / cber.19360690411
- ↑ BC Haynie et al: Matrix Isolation and Density Functional Theory Study of Bis (trifluoromethyl) dioxodiazine: A Photodimer of Trifluoronitrosomethane. In: J Phys Chem A 109, 2005, pp 5307-5315. doi : 10.1021 / jp050730z
- ↑ K. Kuchitsu: CF 3 NO Trifluoronitrosomethane. In: Landolt-Börnstein - Group II Molecules and Radicals 25, 1999, pp. 1615-1852. doi : 10.1007 / 10653318_100
- ↑ a b A. Senning: N-, O-, and S-trihalomethyl compounds. In: Chemical Reviews 65, 1964, pp. 385-412.
- ↑ M. Schmeisser et al.: The plasma-chemical representation of trifluoroiodomethane, bromotrifluoromethane and trifluoronitrosomethane. In: Journal for inorganic and general chemistry 418, 1975, pp. 109-115. doi : 10.1002 / zaac.19754180204
- ↑ CW Taylor et al: The Preparation of Polyfluoronitrosoalkanes from Nitrosyl Polyfluoroacylates. In: The Journal of organic chemistry 27, 1962, pp. 1064-1066. doi : 10.1021 / jo01050a523
- ↑ JD Park et al .: Preparation of Perfluoronitrosoalkanes. Reaction of Trifluoroacetic Anhydride with Nitrosyl Chloride. In: The Journal of organic chemistry 27, 1962, p. 1642. doi : 10.1021 / jo01051a519 .
literature
- Activated carbon impregnated with organic amines Patent DE69420079
- JS Spasov and JI Cline: Scalar and angular correlations in CF 3 NO photodissociation: statistical and nonstatistical channels. In: Journal of Chemical Physics 110, 1999, pp. 9568-9577.
- EO John et al .: Difluorodinitrosomethane, ONCF 2 NO, and Hexafluorodinitrosopropane, ONCF 2 CF 2 CF 2 NO. In: Inorg Chem 31, 1992, pp. 329-331, doi: 10.1021 / ic00028a040 .
- RC White and LJ Parcell: The Photolysis of Trifluoronitrosomethane. In: The Journal of Physical Chemistry 69, 1965, pp. 4409-4410.
- JE Boggs et al: The Dipole Moment of Trifluoronitrosomethane. In: The Journal of Physical Chemistry 68, 1964, pp. 2383-2384.
- MI Davis et al: An Electron Diffraction Study of Trifluoronitrosomethane. In: The Journal of Physical Chemistry 69, 1965, pp. 3727-3730.
Web links
- Landolt-Börnstein (English)