Trifluperidol
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
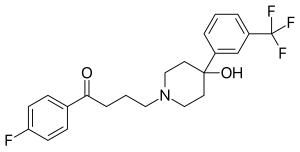
|
|||||||||||||
| General | |||||||||||||
| Surname | Trifluperidol | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 22 H 23 F 4 NO 2 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass |
|
||||||||||||
| Melting point |
|
||||||||||||
| solubility |
soluble in water (hydrochloride) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data |
|
||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Trifluperidol is a chemical compound developed by Janssen Pharmaceutica in 1959 that was used as an antipsychotic . It is one of the butyrophenones . Until its approval under the trade name Triperidol ® expired in 2005, it was the most potent neuroleptic on the European market.
Extraction and presentation
The synthesis of trifluperidol takes place in three steps. In the first step, the Grignard compound of 3-trifluoromethylbromobenzene is reacted with 1-benzyl-4-piperidone. The resulting intermediate is then reductively debenzylated. By reacting 4-hydroxy-4- (3-trifluoromethylphenyl) piperidine with 4-chloro-4'-fluorobutyrophenone, the target compound is obtained in the last step.
The hydrochloride is mostly used in pharmaceutical formulations.
history
Trifluperidol was first synthesized in 1959 and marketed by Janssen Pharmaceutica in 1961 under the name Triperidol ® . Clinical studies from 1960 and 1964, which compared the psychotropic drug with haloperidol , among other things , came to the conclusion that haloperidol is much more suitable for the treatment of schizophrenia . Trifluperidol had too many and serious side effects and was especially ineffective in nergic, withdrawn and disinterested patients.
application areas
Trifluperidol has been used in severe forms of acute and chronic schizophrenia .
literature
- DM Gallant, MP Bishop, E. Timmons, CA Steele: A Controlled Evaluation of Trifluperidol: a New Potent Psychopharmacologic Agent. In: Curr Ther Res Clin Exp . 27 Sep 1963, pp. 463-471. PMID 14065098 .
- DM Gallant, MP Bishop, E. Timmons, CA Steele: Trifluperidol: a butyrophenone derivative. In: Am J Psychiatry . 120, Nov 1963, pp. 485-487. doi: 10.1176 / ajp.120.5.485 . PMID 14051242 .
- Wolfgang Gäbel, Gerd Laux: Biological Psychiatry Synopsis 1990/91
Individual evidence
- ↑ a b c d e f g h i Entry on Trifluperidol. In: Römpp Online . Georg Thieme Verlag, accessed on August 8, 2017.
- ↑ a b data sheet Trifluperidol hydrochloride from Sigma-Aldrich , accessed on August 8, 2017 ( PDF ).
- ^ A b c d A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications. 4th edition. Thieme-Verlag Stuttgart 2001, ISBN 1-58890-031-2 .
- ↑ a b Trifluperidol: medication, active ingredient, application, side effects, risks. In: symptomat.de. Retrieved August 8, 2017 .
- ^ WR Goodchild, BC Schiele, ND Vestre, LM Tredici, R. Zimmermann: A Double Blind Comparison of Trifluperidol and Trifluoperazine in Chronic Schizophrenic Patients . In: International Pharmacopsychiatry . tape 2 , 1969, ISSN 0020-8272 , pp. 185–196 , doi : 10.1159 / 000468852 ( karger.com [accessed July 12, 2020]).
- ↑ F. von Bruchhausen, G. Dannhardt, S. Ebel, AW Frahm, E. Hackenthal, R. Hänsel, U. Holzgrabe, K. Keller, E. Nürnberg, H. Rimpler, G. Schneider, P. Surmann, HU Wolf, G. Wurm (Ed.): Hager's handbook of pharmaceutical practice . 5th edition. Springer-Verlag, Berlin / Heidelberg 2013, ISBN 978-3-642-57880-9 , pp. 1053 ( limited preview in Google Book search).
